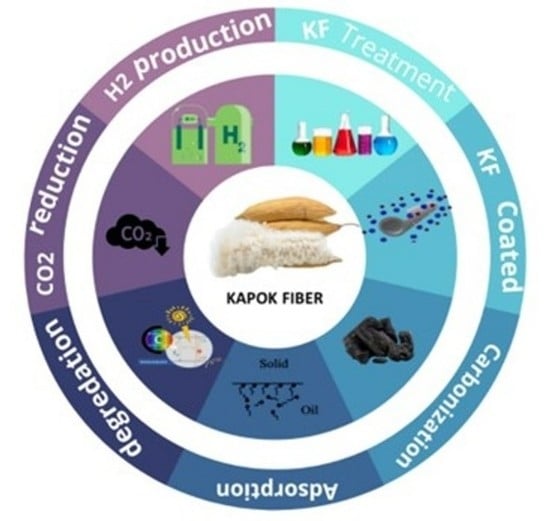
Biobased Kapok Fiber Nano-Structure for Energy and Environment Application: A Critical Review
Abstract
:1. Introduction
2. Biochar as Sustainable Material
3. Overview and Properties of Kapok Fiber
3.1. Overview of Kapok Fiber
3.2. Kapok Fiber Composition
4. Preparation and Treatment of Kapok Fiber and Derived Materials
4.1. Chemical Treatment
4.2. Physical Treatment
4.3. Carbonization Process
4.4. Coated of Kapok Fiber
5. Applications of Kapok Fiber
5.1. Photocatalytic Hydrogen Production

5.2. Photocatalytic Degradation
5.2.1. Mechanism of Photodegradation
5.2.2. Kapok-Based Composite for Degradation
5.3. Adsorption Using KF
5.3.1. The Main Parameters Effect on Kapok Fiber
5.3.2. Kapok Fiber Characterization as Adsorbent
5.4. Photocatalytic CO2 Reduction
6. Conclusions and Future Recommendations
6.1. Conclusions
6.2. Future Recommendation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahir, M.; Tahir, B. Constructing S-scheme 2D/0D g-C3N4/TiO2 NPs/MPs heterojunction with 2D-Ti3AlC2 MAX cocatalyst for photocatalytic CO2 reduction to CO/CH4 in fixed-bed and monolith photoreactors. J. Mater. Sci. Technol. 2022, 106, 195–210. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, M.; Yu, T.; Wang, J. 3D g-C3N4/WO3/biochar/Cu2+-doped carbon spheres composites: Synthesis and visible-light-driven photocatalytic hydrogen production. Mater. Today Commun. 2022, 30, 103084. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Lee, J.H.Q.; Lauw, S.J.L.; Webster, R.D. The electrochemical reduction of carbon dioxide (CO2) to methanol in the presence of pyridoxine (vitamin B6). Electrochem. Commun. 2016, 64, 69–73. [Google Scholar] [CrossRef]
- Martín, A.J.; Larrazábal, G.O.; Pérez-Ramírez, J. Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: Lessons from water electrolysis. Green Chem. 2015, 17, 5114–5130. [Google Scholar] [CrossRef]
- Aguilera, F.; Mendez, J.; Pasaro, E.; Laffon, B. Review on the effects of exposure to spilled oils on human health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Effect of kapok fiber treated with various solvents on oil absorbency. Ind. Crops Prod. 2012, 40, 178–184. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Wang, F. Pore structure of kapok fiber. Cellulose 2018, 25, 3219–3227. [Google Scholar] [CrossRef]
- Herrera, M.U.; Futalan, C.M.; Gapusan, R.; Balela, M.D.L. Removal of methyl orange dye and copper (II) ions from aqueous solution using polyaniline-coated kapok (Ceiba pentandra) fibers. Water Sci. Technol. 2018, 78, 1137–1147. [Google Scholar] [CrossRef]
- Yang, X.; Han, X.; Chen, Z.; Zhou, L.; Zhao, B.; Su, H.; Jiao, W. Fabrication of Li7La3Zr2O12 fibers using bio-mass template Kapok. Mater. Lett. 2018, 217, 271–275. [Google Scholar] [CrossRef]
- An, J.J.L.; Liang, W.; Mu, P.; Wang, C.; Chen, T.; Zhu, Z.; Sun, H.; Li, A. Novel Sugar Alcohol/Carbonized Kapok Fiber Composites as Form-Stable Phase-Change Materials with Exceptionally High Latent Heat for Thermal Energy Storage. ACS Omega 2019, 4, 4848–4855. [Google Scholar] [CrossRef]
- Yu, Z.E.; Lyu, Y.; Wang, Y.; Xu, S.; Cheng, H.; Mu, X.; Chu, J.; Chen, R.; Liu, Y.; Guo, B. Hard carbon micro-nano tubes derived from kapok fiber as anode materials for sodium-ion batteries and the sodium-ion storage mechanism. Chem. Commun. 2020, 56, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kim, D.; Shin, C.-H.; Zhao, Y.; Park, J.-S.; Ryu, M. Removal of lead(II) from aqueous stream by hydrophilic modified kapok fiber using the Fenton reaction. Environ. Earth Sci. 2018, 77, 653. [Google Scholar] [CrossRef]
- Pai, A.R.; Jagtap, R.N. Surface Morphology & Mechanical properties of some unique Natural Fiber Reinforced Polymer Composites—A Review. J. Mater. Environ. Sci. 2015, 6, 902–917. [Google Scholar]
- Zheng, Y.; Wang, J.; Zhu, Y.; Wang, A. Research and application of kapok fiber as an absorbing material: A mini review. J. Environ. Sci. 2015, 27, 21–32. [Google Scholar] [CrossRef]
- Swain, S.R.; Swain, I.; Das, P. Kapok Fiber-Structure, Characteristics and Applications: A Review. IJEMHS 2019, 31, 314–324. [Google Scholar]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- George, W.; Huber, S.I.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; Garcia-Martinez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.; Ribeiro Carrott, M.M. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Lithoxoos, G.P.; Labropoulos, A.; Peristeras, L.D.; Kanellopoulos, N.; Samios, J.; Economou, I.G. Adsorption of N2, CH4, CO and CO2 gases in single walled carbon nanotubes: A combined experimental and Monte Carlo molecular simulation study. J. Supercrit. Fluids 2010, 55, 510–523. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volotskova, O.; Levchenko, I.; Shashurin, A.; Raitses, Y.; Ostrikov, K.; Keidar, M. Single-step synthesis and magnetic separation of graphene and carbon nanotubes in arc discharge plasmas. Nanoscale 2010, 2, 2281–2285. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Shen, J.; Jayaprakash, N.; Archer, L.A. Synthesis of organic–inorganic hybrids by miniemulsion polymerization and their application for electrochemical energy storage. Energy Environ. Sci. 2012, 5, 7025–7032. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Eng. Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.R.; Wright, M.M.; Brown, R.C. Estimating profitability of two biochar production scenarios: Slow pyrolysis vs fast pyrolysis. Biofuels Bioprod. Biorefining 2011, 5, 54–68. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Kong, S.-H.; Loh, S.-K.; Bachmann, R.T.; Rahim, S.A.; Salimon, J. Biochar from oil palm biomass: A review of its potential and challenges. Renew. Sustain. Energy Rev. 2014, 39, 729–739. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts. RSC Adv. 2014, 4, 10731–10737. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Yue, J.; Gao, S.; Xu, G. Catalytic upgrading of coal pyrolysis tar over char-based catalysts. Fuel Process. Technol. 2014, 122, 98–106. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [Green Version]
- Namaalwa, J.; Sankhayan, P.L.; Hofstad, O. A dynamic bio-economic model for analyzing deforestation and degradation: An application to woodlands in Uganda. For. Policy Econ. 2007, 9, 479–495. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Kapok Fiber: Structure and Properties. In Biomass Bioenergy; Springer Nature: Cham, Switzerland, 2014; pp. 101–110. [Google Scholar]
- Zhang, Z.; Zhang, H.; Gao, Y.; Kang, H. Laboratory evaluation of the effect of kapok fibers on the rheological and fatigue properties of bitumen. Constr. Build. Mater. 2021, 272, 121819. [Google Scholar] [CrossRef]
- Macedo, M.J.P.; Silva, G.S.; Feitor, M.C.; Costa, T.H.C.; Ito, E.N.; Melo, J.D.D. Surface modification of kapok fibers by cold plasma surface treatment. J. Mater. Res. Technol. 2020, 9, 2467–2476. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Chaiarrekij, S.; Apirakchaiskul, A.; Suvarnakich, K.; Kiatkamjornwong, S. Characteristcs of kapok fiber as a potential pulp source for papermaking. BioResources 2012, 7, 0475–0488. [Google Scholar]
- Xu, W.; Mu, B.; Wang, A. Facile fabrication of well-defined microtubular carbonized kapok fiber/NiO composites as electrode material for supercapacitor. Electrochim. Acta 2016, 194, 84–94. [Google Scholar] [CrossRef]
- Kiyama, S.; Maeda, S. Security Paper Sheets Using Kapok Fibers Containing. Chromic Materials. J. Print. Sci. Technol. 2017, 1, 023–026. [Google Scholar]
- Lacuesta, A.C.; Herrera, M.U.; Manalo, R.; Balela, M.D.L. Fabrication of kapok paper-zinc oxide-polyaniline hybrid nanocomposite for methyl orange removal. Surf. Coat. Technol. 2018, 350, 971–976. [Google Scholar] [CrossRef]
- Nyankson, E.; Rodene, D.; Gupta, R.B. Advancements in Crude Oil Spill Remediation Research after the Deepwater Horizon Oil Spill. Water Air Soil Pollut. 2015, 227, 1–22. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Duan, C.; Xiao, H.; Shi, M.; Zhao, N.; Xu, J. Superhydrophobicity determines the buoyancy performance of kapok fiber aggregates. Appl. Surf. Sci. 2013, 266, 225–229. [Google Scholar] [CrossRef]
- Xiang, H.-F.; Wang, D.; Liua, H.-C.; Zhao, N.; Xu, J. Investigation on sound absorption properties of kapok fibers. Chin. J. Polym. Sci. 2013, 31, 521–529. [Google Scholar] [CrossRef]
- Reddy, G.V.; Naidu, S.V.; Rani, T.S. Impact Properties of Kapok Based Unsaturated Polyester Hybrid Composites. J. Reinf. Plast. Compos. 2008, 27, 1789–1804. [Google Scholar] [CrossRef]
- Reddy, G.V.; Naidu, S.V.; Rani, T.S. A Study on Hardness and Flexural Properties of Kapok/Sisal Composites. J. Reinf. Plast. Compos. 2009, 28, 2035–2044. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Chaiwatyothin, S.; Mueangta, S.; Hanchana, A. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Superhydrophobic kapok fiber oil-absorbent: Preparation and high oil absorbency. Chem. Eng. J. 2012, 213, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Investigation of acetylated kapok fibers on the sorption of oil in water. J. Environ. Sci. 2013, 25, 246–253. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Preparation and properties of kapok fiber enhanced oil sorption resins by suspended emulsion polymerization. J. Appl. Polym. Sci. 2013, 127, 2184–2191. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Kang, Y.; Wang, A. Investigation of oil sorption capability of PBMA/SiO2 coated kapok fiber. Chem. Eng. J. 2013, 223, 632–637. [Google Scholar] [CrossRef]
- Huang, X.; Lim, T.-T. Performance and mechanism of a hydrophobic–oleophilic kapok filter for oil/water separation. Desalination 2006, 190, 295–307. [Google Scholar] [CrossRef]
- Lim, T.-T.; Huang, X. Evaluation of hydrophobicity/oleophilicity of kapok and its performance in oily water filtration: Comparison of raw and solvent-treated fibers. Ind. Crops Prod. 2007, 26, 125–134. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Rahmah, A.U.; Man, Z. Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. J. Hazard. Mater. 2010, 177, 683–691. [Google Scholar] [CrossRef]
- Rengasamy, R.S.; Das, D.; Karan, C.P. Study of oil sorption behavior of filled and structured fiber assemblies made from polypropylene, kapok and milkweed fibers. J. Hazard. Mater. 2011, 186, 526–532. [Google Scholar] [CrossRef]
- Fan, H.; Yu, X.; Long, Y.; Zhang, X.; Xiang, H.; Duan, C.; Zhao, N.; Zhang, X.; Xu, J. Preparation of kapok–polyacrylonitrile core–shell composite microtube and its application as gold nanoparticles carrier. Appl. Surf. Sci. 2012, 258, 2876–2882. [Google Scholar] [CrossRef]
- Chung, J.-T.; Hwang, K.-J.; Shim, W.-G.; Kim, C.; Park, J.-Y.; Choi, D.-Y.; Lee, J.-W. Synthesis and characterization of activated hollow carbon fibers from Ceiba pentandra (L.) Gaertn. (kapok). Mater. Lett. 2013, 93, 401–403. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Abdullah, W.N.W.; Leh, C.P. Potential of Ceiba pentandra (L.) Gaertn. (kapok fiber) as a resource for second generation bioethanol: Effect of various simple pretreatment methods on sugar production. Bioresour. Technol. 2012, 116, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.W.; Jaafar, J.; Ismail, A.; Mutalib, M.A.; Mohamad, A.B.; Zain, M.M.; Awang, N.A.; Hir, Z.A.M. Physicochemical characterization of cellulose nanocrystal and nanoporous self-assembled CNC membrane derived from Ceiba pentandra. Carbohydr. Polym. 2017, 157, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chen, X.; He, Y.; Zhou, J.; Zhao, H.; Chen, W.; Wang, J.; Wang, X. Fabrication of kapok fibers and natural rubber composites for pressure sensor applications. Cellulose 2021, 28, 2287–2301. [Google Scholar] [CrossRef]
- Nilsson, T.; Björdal, C. The use of kapok fibres for enrichment cultures of lignocellulose-degrading bacteria. Int. Biodeterior. Biodegrad. 2008, 61, 11–16. [Google Scholar] [CrossRef]
- Chung, B.-Y.; Cho, J.-Y.; Lee, M.-H.; Wi, S.-G.; Kim, J.-H.; Kim, J.-S.; Kang, P.-H.; Nho, Y.-C. Adsorption of Heavy Metal Ions onto Chemically Oxidized Ceiba pentandra (L.) Gaertn. (Kapok) Fibers. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 28–35. [Google Scholar] [CrossRef]
- Dauda, B.M.D.; Kolawole, E.G. Processibility of Nigerian kapok fibre. Indian J. Fibre Text. Res. 2003, 28, 147–149. [Google Scholar]
- Lou, L. Structure, properties of kapok fiber and test on heat retention. Shanghai Text Sci. Technol. 2011, 39, 15–17. [Google Scholar]
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Ngaini, Z.; Nourouzi, M.M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F. Effect of alkali treatment on structure and properties of yarn containing kapok fiber. J. Text. Res. 2009, 30, 55–60. [Google Scholar]
- Liu, J.; Wang, F. Influence of Mercerization on Micro-structure and Properties of Kapok Blended Yarns with Different Blending Ratios. J. Eng. Fibers Fabr. 2011, 6, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Park, E.A.; Seo, H.; Lee, S.-Y. Zeolite-fiber membrane enabling low-temperature combustion and high reduction of liquid wastes. Mater. Lett. 2021, 291, 129513. [Google Scholar] [CrossRef]
- Lim, T.-T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic-oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B.; Bisoyi, D.K. Synthesis and characterization of kapok fibers and its composites. J. Appl. Sci. 2012, 12, 1661–1665. [Google Scholar] [CrossRef] [Green Version]
- Phil Hyun Kang, J.P.J.; Chung, B.Y.; Kim, J.S.; Nho, Y.C. Preparation and Characterization of Glycidyl Methacrylate (GMA) Grafted Kapok Fiber by Using Radiation Induced-grafting Technique. J. Ind. Eng. Chem. 2007, 13, 956–958. [Google Scholar]
- Liu, Y.; Wang, J.; Zheng, Y.; Wang, A. Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chem. Eng. J. 2012, 184, 248–255. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A. Acetylated modification of kapok fiber and application for oil absorption. Fibers Polym. 2013, 14, 1834–1840. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Chen, B.; Wu, K.; Nan, B.; Lu, M.; Lu, M. PEG-filled kapok fiber/sodium alginate aerogel loaded phase change composite material with high thermal conductivity and excellent shape stability. Compos. Part A 2021, 143, 106279. [Google Scholar] [CrossRef]
- Marwanto, M.; Maulana, M.I.; Febrianto, F.; Wistara, N.J.; Nikmatin, S.; Masruchin, N.; Zaini, L.H.; Lee, S.H.; Kim, N.H. Effect of Oxidation Time on the Properties of Cellulose Nanocrystals Prepared from Balsa and Kapok Fibers Using Ammonium Persulfate. Polymers 2021, 13, 1894. [Google Scholar] [CrossRef] [PubMed]
- Wolok, E.; Lahay, I.H.; Machmoed, B.R.; Pakaya, F. Modification and characterization of Ceiba Pentandra (L.) gaertn. (kapok) fiber: Physical properties. Int. J. Res. 2019, 7, 381–390. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Y.; Zhao, W.; Ye, W.; Zhou, S. Porous hollow carbon tube derived from kapok fibres as efficient metal-free oxygen reduction catalysts. Sci. China Technol. Sci. 2019, 62, 1710–1718. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, E.; Tu, L.; Wang, A.; Hu, H. A comparative study for oil-absorbing performance of octadecyltrichlorosilane treated Calotropis gigantea fiber and kapok fiber. Cellulose 2017, 24, 989–1000. [Google Scholar] [CrossRef]
- Hina, K.; Zou, H.; Qian, W.; Zuo, D.; Yi, C. Preparation and performance comparison of cellulose-based activated carbon fibres. Cellulose 2018, 25, 607–617. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Huang, X.; Wang, L. Metal (Sn, Bi, Pb, Cd) in-situ anchored on mesoporous hollow kapok-tubes for outstanding electrocatalytic CO2 reduction to formate. Electrochim. Acta 2019, 325, 989–1000. [Google Scholar] [CrossRef]
- Song, P.; Cui, J.; Di, J.; Liu, D.; Xu, M.; Tang, B.; Zeng, Q.; Xiong, J.; Wang, C.; He, Q.; et al. Carbon Microtube Aerogel Derived from Kapok Fiber: An Efficient and Recyclable Sorbent for Oils and Organic Solvents. ACS Nano 2020, 14, 595–602. [Google Scholar] [CrossRef]
- Wang, J.-R.; Wan, F.; Lü, Q.-F.; Chen, F.; Lin, Q. Self-nitrogen-doped porous biochar derived from kapok (Ceiba insignis) fibers: Effect of pyrolysis temperature and high electrochemical performance. J. Mater. Sci. Technol. 2018, 34, 1959–1968. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Lu, L.; Yu, W.; Wang, Y.; Zhang, K.; Zhu, X.; Zhang, Y.; Wu, Y.; Ullah, H.; Xiao, X.; Chen, B. Application of biochar-based materials in environmental remediation: From multi-level structures to specific devices. Biochar 2020, 2, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liu, X.; Jiang, Y.; Lin, D.; Yang, K. Sorption kinetics of 1,3,5-trinitrobenzene to biochars produced at various temperatures. Biochar 2022, 4, 32. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Meiwu, S.; Hong, X.; Weidong, Y. The Fine Structure of the Kapok Fiber. Text. Res. J. 2009, 80, 159–165. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Wang, M.; Tafti, N.D.; Wang, J.J.; Wang, X. Effect of pyrolysis temperature on Si release of alkali-enhanced Si-rich biochar and plant response. Biochar 2021, 3, 469–484. [Google Scholar] [CrossRef]
- Angin, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Xu, W.; Mu, B.; Wang, A. Three-dimensional hollow microtubular carbonized kapok fiber/cobalt-nickel binary oxide composites for high-performance electrode materials of supercapacitors. Electrochim. Acta 2017, 224, 113–124. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Sun, H.Y.; Zhuang, J.; Lin, L.; Ouyang, P. Clean conversion of cellulose into fermentable glucose. Biotechnol. Adv. 2009, 27, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ahn, H.J.; Wang, G. SnO2@graphene nanocomposites as anode materials for Na-ion batteries with superior electrochemical performance. Chem. Commun. Camb. 2013, 49, 3131–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Agcaoili, A.R.; Herrera, M.U.; Futalan, C.M.; Balela, M.D.L. Fabrication of polyacrylonitrile-coated kapok hollow microtubes for adsorption of methyl orange and Cu(II) ions in aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 78, 359–369. [Google Scholar] [CrossRef]
- Guojian, C.; Agcaoili, A.; Ishihara, K.; Balela, M.D.; Muhammad, Y. Preparation of Polyacrylonitrile-Kapok Hollow Microtubes Decorated with Cu Nanoparticles. MATEC Web Conf. 2015, 27, 02008. [Google Scholar]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- AlZahrani, A.A.; Dincer, I. Modeling and performance optimization of a solid oxide electrolysis system for hydrogen production. Appl. Energy 2018, 225, 471–485. [Google Scholar] [CrossRef]
- Swaren, L.; Safari, S.; Konhauser, K.O.; Alessi, D.S. Pyrolyzed biomass-derived nanoparticles: A review of surface chemistry, contaminant mobility, and future research avenues to fill the gaps. Biochar 2022, 4, 33. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Ouyang, L.; Shao, H.; Zhu, M. Promoting hydrogen generation from the hydrolysis of Mg-Graphite composites by plasma-assisted milling. Energy 2019, 167, 1205–1211. [Google Scholar] [CrossRef]
- Chen, K.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Zhang, Y.; Zhu, M. Converting H+ from coordinated water into H− enables super facile synthesis of LiBH4. Green Chem. 2019, 21, 4380–4387. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage. Adv. Eng. Mater. 2017, 7, 1700299. [Google Scholar] [CrossRef]
- Tan, Z.H.; Ouyang, L.Z.; Huang, J.M.; Liu, J.W.; Wang, H.; Shao, H.Y.; Zhu, M. Hydrogen generation via hydrolysis of Mg2Si. J. Alloys Compd. 2019, 770, 108–115. [Google Scholar] [CrossRef]
- Shi, J.; Feng, S.; Chen, T.; Liu, Z.; Yue, X. High-efficiency visible light photocatalytic performances of the CdS(HS)/g-C3N4 composites: The role of intimate connection and hollow structure. J. Mater. Sci. Mater. Electron. 2019, 30, 10867–10878. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Symes, M.D.; Cronin, L. Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 2013, 5, 403–409. [Google Scholar] [CrossRef]
- Sun, H.S.; Sun, M.; Fang, Y.; Wang, Y.; Wang, H. One-step in situ calcination synthesis of g-C3N4/N-TiO2 hybrids with enhanced photoactivity. RSC Adv. 2016, 6, 13063–13071. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B 2019, 257, 117855. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Shi, Z.-L.; Du, C.; Yao, S.-H. Preparation and photocatalytic activity of cerium doped anatase titanium dioxide coated magnetite composite. J. Taiwan Inst. Chem. Eng. 2011, 42, 652–657. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Jaafar, J.; Amin, N.A.S.; Ng, Y.H. Revealing the role of kapok fibre as bio-template for In-situ construction of C-doped g-C3N4@C, N co-doped TiO2 core-shell heterojunction photocatalyst and its photocatalytic hydrogen production performance. Appl. Surf. Sci. 2019, 476, 205–220. [Google Scholar] [CrossRef]
- Mohamed, M.A.M.A.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Amin, N.A.S.; Salleh, W.N.W.; Salehmin, M.N.I.; Nasir, M.F.M.; Hir, Z.A.M. Constructing bio-templated 3D porous microtubular C-doped g-C3N4 with tunable band structure and enhanced charge carrier separation. Appl. Catal. B 2018, 236, 265–279. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Huang, S.; Huang, X.; Xu, B.; Hu, L.; Cui, H.; Ruan, S.; Zeng, Y.-J. Bio-inspired carbon doped graphitic carbon nitride with booming photocatalytic hydrogen evolution. Appl. Catal. B 2019, 246, 61–71. [Google Scholar] [CrossRef]
- Kong, L.; Ji, Y.; Dang, Z.; Yan, J.; Li, P.; Li, Y.; Liu, S.F. g-C3N4 Loading Black Phosphorus Quantum Dot for Efficient and Stable Photocatalytic H2 Generation under Visible Light. Adv. Funct. Mater. 2018, 28, 1800668. [Google Scholar] [CrossRef]
- Wang, A.F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K.; et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Kang, Y.; Wang, A. Au nanoparticles decorated Kapok fiber by a facile noncovalent approach for efficient catalytic decoloration of Congo Red and hydrogen production. Chem. Eng. J. 2014, 237, 336–343. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Sun, G.; Su, F.; Kong, Q.-Q.; Li, F.; Ma, W.; Shi, J.; Jiang, D.; Lu, C.; et al. Hollow carbon microtubes from kapok fiber: Structural evolution and energy storage performance. Sustain. Energy Fuels 2018, 2, 455–465. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Qiang, W.; Xu, J.; Xu, D. Enzyme-free colorimetric bioassay based on gold nanoparticle-catalyzed dye decolorization. Analyst 2013, 138, 760–766. [Google Scholar] [CrossRef]
- Azad, U.P.; Ganesan, V.; Pal, M. Catalytic reduction of organic dyes at gold nanoparticles impregnated silica materials: Influence of functional groups and surfactants. J. Nanopart. Res. 2011, 13, 3951–3959. [Google Scholar] [CrossRef]
- Azad Kumar, D.K.; Pandey, G. Characterisation of hydrothermally synthesised cuo nanoparticles at different pH. J. Technol. Adv. Sci. Res. 2016, 2, 166–169. [Google Scholar]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Enhanced photocatalytic activity of bismuth-doped TiO2 nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye. J. Nanopart. Res. 2013, 15, 1669. [Google Scholar] [CrossRef]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Abou-Gamra, Z.M.; Ahmed, M.A. Synthesis of mesoporous TiO2-curcumin nanoparticles for photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B 2016, 160, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, W.; Qiu, J.; Li, J.; Zhou, W.; Fang, Y.; Zhang, S.; Li, X. Enhanced photocatalytic degradation and adsorption of methylene blue via TiO2 nanocrystals supported on graphene-like bamboo charcoal. Appl. Surf. Sci. 2015, 358, 425–435. [Google Scholar] [CrossRef]
- Hamad, H.A.; Sadik, W.A.; El-Latif, M.M.A.; Kashyout, A.B.; Feteha, M.Y. Photocatalytic parameters and kinetic study for degradation of dichlorophenol-indophenol (DCPIP) dye using highly active mesoporous TiO2 nanoparticles. J. Environ. Sci. 2016, 43, 26–39. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic activities of the nano-sized TiO2-supported Y-zeolites. J. Photochem. Photobiol. C 2003, 4, 5–18. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Guo, X.; Ding, Q.; Pullerits, T.; Wang, R.; Zhang, G.; Liang, W.; Sun, M. Plasmon-exciton coupling of monolayer MoS2-Ag nanoparticles hybrids for surface catalytic reaction. Mater. Today Energy 2017, 5, 72–78. [Google Scholar] [CrossRef]
- Challagulla, S.; Nagarjuna, R.; Ganesan, R.; Roy, S. TiO2 synthesized by various routes and its role on environmental remediation and alternate energy production. Nano-Struct. Nano-Objects 2017, 12, 147–156. [Google Scholar] [CrossRef]
- Arques, A.; Amat, A.M.; Santos-Juanes, L.; Vercher, R.F.; Marín, M.L.; Miranda, M.A. 2,4,6-Triphenylthiapyrylium cation as homogeneous solar photocatalyst. Catal. Today 2007, 129, 37–42. [Google Scholar] [CrossRef]
- Hu, H.S.; Wang, H.; Wang, F.; Li, Z.; Li, W.; Bai, J. Synthesis of kappa fiber modified graphite carbon nitride with outstanding photocatalytic phenol degradation ability. Diam. Relat. Mater. 2020, 105, 107817. [Google Scholar] [CrossRef]
- Zhou, J.; Du, E.; He, Y.; Fan, Y.; Ye, Y.; Tang, B. Preparation of Carbonized Kapok Fiber/Reduced Graphene Oxide Aerogel for Oil-Water Separation. Chem. Eng. Technol. 2020, 43, 2418–2427. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Wang, A. Kapok Fiber Oriented Polyaniline for Removal of Sulfonated Dyes. Ind. Eng. Chem. Res. 2012, 51, 10079–10087. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Huang, D.; Wang, A. Kapok fiber oriented-polyaniline nanofibers for efficient Cr(VI) removal. Chem. Eng. J. 2012, 191, 154–161. [Google Scholar] [CrossRef]
- Purnawati, R.; Febrianto, F.; Wistara, I.N.J.; Nikmatin, S.; Hidayat, W.; Lee, S.H.; Kim, N.H. Physical and Chemical Properties of Kapok (Ceiba pentandra) and Balsa (Ochroma pyramidale) Fibers. J. Korean Wood Sci. Technol. 2018, 46, 393–401. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Gapusan, R.B.; Balela, M.D.L. Adsorption of anionic methyl orange dye and lead(II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater. Chem. Phys. 2019, 243, 122682. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Pushpa, T.B.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis. and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Deschamps, G.; Caruel, H.; Borredon, M.-E.; Bonnin, C.; Vignoles, C. Oil Removal from Water by Selective Sorption on Hydrophobic Cotton Fibers. 1. Study of Sorption Properties and Comparison with Other Cotton Fiber-Based Sorbents. Environ. Sci. Technol. 2003, 37, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Konno, H.; Toyoda, M.; Moriya, K.; Kihara, T. Sorption and recovery of heavy oils by using exfoliated graphite Part II: Recovery of heavy oil and recycling of exfoliated graphite. Desalination 2000, 128, 213–218. [Google Scholar] [CrossRef]
- Bastani, D.; Safekordi, A.A.; Alihosseini, A.; Taghikhani, V. Study of oil sorption by expanded perlite at 298.15K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Teas, C.; Kalligeros, S.; Zanikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G. Anastopoulos Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination 2001, 140, 259–264. [Google Scholar] [CrossRef]
- Mysore, D.; Viraraghavan, T.; Jin, Y.C. Treatment of oily waters using vermiculite. Water Res. 2005, 39, 2643–2653. [Google Scholar] [CrossRef]
- Carmody, O.; Frost, R.; Xi, Y.; Kokot, S. Adsorption of hydrocarbons on organo-clays—Implications for oil spill remediation. J. Colloid Interface Sci. 2007, 305, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Viraraghavan, H.M.A.T. Coalescence/Filtration of an Oil-In-Water Emulsion in a Granular Organo-Clay/Anthracite Mixture Bed, Water, Air. Soil Pollut. 2002, 138, 253–270. [Google Scholar]
- Banerjee, S.S.; Joshi, M.V.; Jayaram, R.V. Treatment of oil spills using organo-fly ash. Desalination 2006, 195, 32–39. [Google Scholar] [CrossRef]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Mar. Pollut. Bull. 2003, 46, 780–783. [Google Scholar] [CrossRef]
- Essawy, H.A.; Essa, M.M.; Abdeen, Z. Oil-absorptive polymeric networks based on dispersed oleophilized nanolayers of laponite within ethylene-propylene-diene monomer vulcanizates. J. Appl. Polym. Sci. 2010, 115, 385–392. [Google Scholar] [CrossRef]
- Xu, B.N.; Xiao, C. A Novel Absorptive Functional Fiber Copolymerized by Butyl Methacrylate with Hydroxyethyl Methacrylate: Preparation and Characterization. Polym.-Plast. Technol. Eng. 2009, 49, 95–103. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Suni, S.; Kosunen, A.L.; Hautala, M.; Pasila, A.; Romantschuk, M. Use of a by-product of peat excavation, cotton grass fibre, as a sorbent for oil-spills. Mar. Pollut. Bull. 2004, 49, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Hyung-Min Choi, J.P.M. Oil sorption behavior of various sorbents studied by sorption capacity measurement and environmental scanning electron microscopy. Microsc. Res. Tech. 1993, 25, 447–455. [Google Scholar] [CrossRef]
- Matuana, L.M.; Balatinecz, J.J.; Sodhi, R.N.; Park, C.B. Park Surface characterization of esterified cellulosic fibers by XPS and FTIR Spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. [Google Scholar] [CrossRef]
- Balela, M.D.L.; Intila, N.M.; Salvanera, S.R. Adsorptive Removal of Lead Ions in Aqueous Solution by Kapok-Polyacrylonitrile Nanocomposites. Mater. Today Proc. 2019, 17, 672–678. [Google Scholar] [CrossRef]
- Duan, C.; Zhao, N.; Yu, X.; Zhang, X.; Xu, J. Chemically modified kapok fiber for fast adsorption of Pb2+, Cd2+, Cu2+ from aqueous solution. Cellulose 2013, 20, 849–860. [Google Scholar] [CrossRef]
- Dong, T.; Cao, S.; Xu, G. Highly efficient and recyclable depth filtrating system using structured kapok filters for oil removal and recovery from wastewater. J. Hazard. Mater. 2017, 321, 859–867. [Google Scholar] [CrossRef]
- Yang, N.; Shin, C.-H.; Kim, D.; Park, J.-S.; Rao, P.; Wang, R. Synthesis, characterization, and mercury removal application of surface modified kapok fibers with dopamine (DA): Investigation of bidentate adsorption. Environ. Earth Sci. 2020, 79, 264. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Surface modifications of natural fibers and performance of the resulting biocomposites: An overview. Compos. Interfaces 2012, 8, 313–343. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. The effect of chemical treatment on the properties of hemp, sisal, jute and kapok for composite reinforcement. Die Angew. Makromol. Chem. 1999, 272, 108–116. [Google Scholar] [CrossRef]
- Ael, A.S.; Ludwick, A.G.; Aglan, H.A. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components. Bioresour. Technol. 2009, 100, 2219–2222. [Google Scholar]
- Saarela, T.; Lafdani, E.K.; Laurén, A.; Pumpanen, J.; Palviainen, M. Biochar as adsorbent in purification of clear-cut forest runoff water: Adsorption rate and adsorption capacity. Biochar 2020, 2, 227–237. [Google Scholar] [CrossRef]
- Tahir, M.; Sherryna, A.; Khan, A.A.; Madi, M.; Zerga, A.Y.; Tahir, B. Defect Engineering in Graphitic Carbon Nitride Nanotextures for Energy Efficient Solar Fuels Production: A Review. Energy Fuels 2022, 36, 8948–8977. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Kiani, G.; Khataee, A. Adsorptive capacity of polyacrylonitrile modified with triethylenetetramine for removal of copper and cadmium ions from aqueous solutions. Environ. Prog. Sustain. Energy 2013, 33, 1139–1147. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, W.; Xu, M.; Wang, J.; Li, Y.; Zhang, Z.; Wang, Y.; Zheng, L.; Li, Q.; Yun, J.; et al. Facile synthesis of oil adsorbent carbon microtubes by pyrolysis of plant tissues. J. Mater. Sci. 2019, 54, 9352–9361. [Google Scholar] [CrossRef]
- Shultz, J.M.; Walsh, L.; Garfin, D.R.; Wilson, F.E.; Neria, Y. The 2010 Deepwater Horizon Oil Spill: The Trauma Signature of an Ecological Disaster. J. Behav. Health Serv. Res. 2014, 42, 58–76. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Z.; Xu, M.; Lu, Y.; Li, Z. 1D SnO2 with Wire-in-Tube Architectures for Highly Selective Electrochemical Reduction of CO2 to C1 Products. Adv. Funct. Mater. 2018, 28, 1706289. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Yue, G.; Hu, Q.; Wang, L. Boosting CH3OH Production in Electrocatalytic CO2 Reduction over Partially Oxidized 5 nm Cobalt Nanoparticles Dispersed on Single-Layer Nitrogen-Doped Graphene. ACS Appl. Mater. Interfaces 2018, 10, 44403–44414. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Hierarchical Mesoporous SnO2 Nanosheets on Carbon Cloth: A Robust and Flexible Electrocatalyst for CO2 Reduction with High Efficiency and Selectivity. Angew. Chem. Int. Ed. Engl. 2017, 56, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Xue, M.; Williams, T.; Zhang, Y.; MacFarlane, D.R.; Zhang, J. Towards a better Sn: Efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets. Nano Energy 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Structured clay minerals-based nanomaterials for sustainable photo/thermal carbon dioxide conversion to cleaner fuels: A critical review. Sci. Total Environ. 2022, 845, 157206. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.F.; Peng, H.; You, C.; Fu, Z.; Huang, P.; Song, H.; Liao, S. High-Performance Doped Carbon Catalyst Derived from Nori Biomass with Melamine Promoter. Electrochim. Acta 2014, 138, 353–359. [Google Scholar] [CrossRef]
- Song, H.; Xu, S.; Li, Y.; Dai, J.; Gong, A.; Zhu, M.; Zhu, C.; Chen, C.; Chen, Y.; Yao, Y.; et al. Hierarchically Porous, Ultrathick, “Breathable” Wood-Derived Cathode for Lithium-Oxygen Batteries. Adv. Eng. Mater. 2017, 8, 1701203. [Google Scholar] [CrossRef]
- Zhu, C.; Du, L.; Luo, J.; Tang, H.; Cui, Z.; Song, H.; Liao, S. A renewable wood-derived cathode for Li–O2 batteries. J. Mater. Chem. A 2018, 6, 14291–14298. [Google Scholar] [CrossRef]























| Composition | Percentage (%) | |
|---|---|---|
| Cellulose | 35 | [17] |
| Hemicellulose | - | |
| Lignin | 21.5 | |
| Holocellulose | 84 | |
| Ash | 1.05 | |
| Wax | 2.34 | |
| Moisture | 11.23 | |
| Xylan | 22 | |
| Acetyl Group | - |
| Materials | Pollutants | Adsorption Results | References |
|---|---|---|---|
| Polyaniline-kapok fiber-nanocomposite | Anionic-methyl-orange | 136.75 mg/g | [146] |
| Kapok fiber | Methylene blue | 110.13 mg/g | [80] |
| Polyaniline-kapok fiber-nanocomposite | Lead ions | 78.34 mg/g | [168] |
| Polyacrylonitrile-coated-kapok hollow-microtube | methyl-orange & Cu (II) ions | 34.72/90.09 mg/g | [105] |
| Kapok fiber-oriented polyaniline | Sulfonated dyes | 192.3 mg/g | [147] |
| Kapok fiber-oriented polyaniline-nanofiber | Cu (II) ions | 145.54 mg/g | [147] |
| Polyaniline-coated kapok fiber | Methyl-orange & copper (II) ions | 81.04 mg/g | [10] |
| Hydrophilic modified kapok fiber | Lead(II) | 94.41 mg/g | [14] |
| Acetylated modification kapok fiber | Oil | 84.4 g/g | [81] |
| Oxidized kapok fiber | Pb, Cu, Cd and Zn | 93.55%, 91.83%, 89.75% and 92.85% | [69] |
| Kapok fiber-based carbon microtube aerogel | Oil/organic solvents | 98% (distillation) 97% (Squeezing) 90% (Combustion) | [89] |
| DTPA-modified kapok fiber | Pb+2, Cd+2, Cu+2 | 310.6 mg/g, 163.7 mg/g, 101 mg/g | [169] |
| Kapok fiber | Diesel | 45 g/g | [77] |
| Kapok fiber | Oil | 32.31 g/g | [170] |
| Raw kapok fiber/pyridine-catalyzed kapok Fiber/NBS-catalyzed kapok fiber | Diesel | 30.5 g/g 36.7 g/g 34 g/g | [56] |
| PBMA/SiO2 | Diesel, Soybean oil, Crude oil, 150SN, 20CST | 99.7%, 65%, 41.1%, 23.1% and 26.8% | [58] |
| PBMA-Kapok Fiber | Toluene and chloroform | 14.6 g/g and 26 g/g | [57] |
| Superhydrophobic—Kapok Fiber | Diesel and Soybean oil | 46.9 g/g and 58.8 | [55] |
| Kapok Fiber—Dopamine | Mercury | 235.7 mg/g | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerga, A.Y.; Tahir, M. Biobased Kapok Fiber Nano-Structure for Energy and Environment Application: A Critical Review. Molecules 2022, 27, 8107. https://doi.org/10.3390/molecules27228107
Zerga AY, Tahir M. Biobased Kapok Fiber Nano-Structure for Energy and Environment Application: A Critical Review. Molecules. 2022; 27(22):8107. https://doi.org/10.3390/molecules27228107
Chicago/Turabian StyleZerga, Abdelmoumin Yahia, and Muhammad Tahir. 2022. "Biobased Kapok Fiber Nano-Structure for Energy and Environment Application: A Critical Review" Molecules 27, no. 22: 8107. https://doi.org/10.3390/molecules27228107
APA StyleZerga, A. Y., & Tahir, M. (2022). Biobased Kapok Fiber Nano-Structure for Energy and Environment Application: A Critical Review. Molecules, 27(22), 8107. https://doi.org/10.3390/molecules27228107