Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline
Abstract
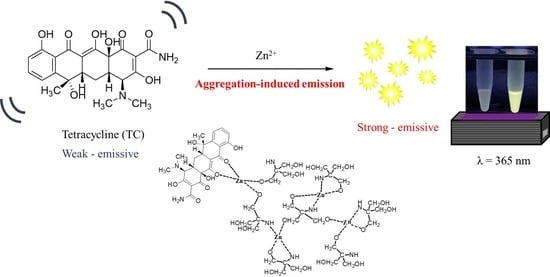
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Examination of TC Fluorescence Enhancement in the Presence of Zn2+
2.4. Optimization of the Experimental Factors
2.5. Using Zn2+ as the Sensing Probe against TC
2.6. Examination of Interference Effects
2.7. Analysis of Simulated Real Samples
3. Results and Discussion
3.1. Characterization of Tris-Zn2+-TC Conjugates
3.2. Optimization of the Experimental Parameters
3.3. Examination of Quantitative Analysis
3.4. Examination of the Effects from Interferences
3.5. Examination of the Precision and Accuracy of the Developed Method
3.6. Analysis of Simulated Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, L.; Yang, W.; He, X.; Zhao, S.; Zhang, X.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Amino-Functionalized Al–MOF for Fluorescent Detection of Tetracyclines in Milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-H.; Zhang, W.; Zheng, Y.; Zhang, W. Correlation among extracellular polymeric substances, tetracycline resistant bacteria and tetracycline resistance genes under trace tetracycline. Chemosphere 2014, 117, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, F.; Mirlohi, M.; Jalali, M.; Azadbakht, L. Dietary exposure to tetracycline residues through milk consumption in Iran. J. Environ. Health Sci. Eng. 2015, 13, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinquina, A.; Longo, F.; Anastasi, G.; Giannetti, L.; Cozzani, R. Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. J. Chromatogr. A 2003, 987, 227–233. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, Y.S.; Niazi, J.H.; Gu, M.B. Electrochemical aptasensor for tetracycline detection. Bioprocess Biosyst. Eng. 2010, 33, 31–37. [Google Scholar] [CrossRef]
- Townshend, A.; Ruengsitagoon, W.; Thongpoon, C.; Liawruangrath, S. Flow injection chemiluminescence determination of tetracycline. Anal. Chim. Acta. 2005, 541, 103–109. [Google Scholar] [CrossRef]
- Jeon, M.; Paeng, I.R. Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay. Anal. Chim. Acta. 2008, 626, 180–185. [Google Scholar] [CrossRef]
- Ramezani, M.; Danesh, N.M.; Lavaee, P.; Abnous, K.; Taghdisi, S.M. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens. Bioelectron. 2015, 70, 181–187. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Gao, J.; Tang, Y.; Wang, Q. Tetracycline generated red luminescence based on a novel lanthanide functionalized layered double hydroxide nanoplatform. J. Agric. Food Chem. 2019, 67, 3871–3878. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.; Zhang, X.; Han, Y.; Tang, W.; Yue, T.; Li, Z. A 3D hierarchical dual-metal–organic framework heterostructure up-regulating the pre-concentration effect for ultrasensitive fluorescence detection of tetracycline antibiotics. J. Mater. Chem. C 2020, 8, 2054–2064. [Google Scholar] [CrossRef]
- Li, P.; Kumar, S.; Park, K.S.; Park, H.G. Development of a rapid and simple tetracycline detection system based on metal-enhanced fluorescence by europium-doped AgNP@ SiO 2 core–shell nanoparticles. RSC Adv. 2018, 8, 24322–24327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Zhang, X.; Xu, C.; Wu, R.; Li, N.; Wang, L.; Zhang, D.; Bi, S.; Fan, Y. Two novel Zn-CPs act as dual-functional luminescent sensors for highly selective, sensitive and stable detection of Fe3+ ions and tetracycline. Inorg. Chim. Acta 2020, 509, 119665. [Google Scholar] [CrossRef]
- Wu, W.-J.; Zhao, Q.; Zhou, R.; Liang, Y.-C.; Zhao, W.-B.; Shan, C.-X. Ratiometric fluorescence sensor based on europium-grafted ZnO quantum dots for visual and colorimetric detection of tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119901. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta. 2019, 1063, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L. Fluorescence Probe Based on Hybrid Mesoporous Silica/Quantum Dot/Molecularly Imprinted Polymer for Detection of Tetracycline. ACS Appl. Mater. Interfaces 2016, 8, 16248–16256. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Li, Y.; Gan, F. Sensitive and selective determination of tetracycline in milk based on sulfur quantum dot probes. RSC Adv. 2021, 11, 22960–22968. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Yang, T.; Wang, J.; Liu, X.D.; Huang, C.Z. One-pot carbonization synthesis of europium-doped carbon quantum dots for highly selective detection of tetracycline. Methods Appl. Fluoresc. 2017, 5, 015003. [Google Scholar] [CrossRef]
- Li, C.; Zeng, C.; Chen, Z.; Jiang, Y.; Yao, H.; Yang, Y.; Wong, W.-T. Luminescent lanthanide metal-organic framework test strip for immediate detection of tetracycline antibiotics in water. J. Hazard. Mater. 2020, 384, 121498. [Google Scholar] [CrossRef]
- Liu, Q.; Ning, D.; Li, W.-J.; Du, X.-M.; Wang, Q.; Li, Y.; Ruan, W.-J. Metal–organic framework-based fluorescent sensing of tetracycline-type antibiotics applicable to environmental and food analysis. Analyst 2019, 144, 1916–1922. [Google Scholar] [CrossRef]
- Fan, C.; Xu, C.; Zhu, B.; Wang, L.; Zong, Z.; Wu, R.; Zhang, X.; Fan, Y. New topological Zn metal organic frameworks as multi-responsive fluorescent sensing materials for detecting Fe3+, Cr2O72−, CrO42− and tetracycline in aqueous system. J. Solid State Chem. 2021, 298, 122157. [Google Scholar] [CrossRef]
- Li, X.; Fan, K.; Yang, R.; Du, X.; Qu, B.; Miao, X.; Lu, L. A long lifetime ratiometrically luminescent tetracycline nanoprobe based on Ir (III) complex-doped and Eu3+-functionalized silicon nanoparticles. J. Hazard. Mater. 2020, 386, 121929. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Huang, P.; Wu, F.-Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, S.; Dou, Y.; Zhuo, Y.; Luo, Y.; Feng, Y. Novel and remarkable enhanced-fluorescence system based on gold nanoclusters for detection of tetracycline. Talanta 2014, 122, 36–42. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Zhang, X.; Huang, Y. Ratiometric detection of tetracycline based on gold nanocluster enhanced Eu3+ fluorescence. Talanta 2020, 206, 120202. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Tan, H.; Chen, Y. Silver nanoparticle enhanced fluorescence of europium (III) for detection of tetracycline in milk. Sens. Actuators B Chem. 2012, 173, 262–267. [Google Scholar] [CrossRef]
- Han, L.; Fan, Y.Z.; Qing, M.; Liu, S.G.; Yang, Y.Z.; Li, N.B.; Luo, H.Q. Smartphones and Test Paper-Assisted Ratiometric Fluorescent Sensors for Semi-Quantitative and Visual Assay of Tetracycline Based on the Target-Induced Synergistic Effect of Antenna Effect and Inner Filter Effect. ACS Appl. Mater. Interfaces 2020, 12, 47099–47107. [Google Scholar] [CrossRef]
- Leng, F.; Zhao, X.J.; Wang, J.; Li, Y.F. Visual detection of tetracycline antibiotics with the turned on fluorescence induced by a metal–organic coordination polymer. Talanta 2013, 107, 396–401. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Zhang, Q.; Li, S.; Lu, X. Tetracycline antibiotics and NH4+ detection by Zn–organic framework fluorescent probe. Analyst 2021, 146, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.; Lambs, L.; Berthon, G. Metal ion-tetracycline interactions in biological fluids. Part 5. Formation of zinc complexes with tetracycline and some of its derivatives and assessment of their biological significance. Agents Actions 1985, 17, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Doluisio, J.T.; Martin, A.N. Metal complexation of the tetracycline hydrochlorides. J. Med. Chem. 1963, 6, 16–20. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Z.; Qian, J.; Zhou, H.; Zhang, K. Copper doped zinc sulfide quantum dots as ratiometric fluorescent probes for rapid and specific detection of tetracycline residues in milk. Anal. Chim. Acta. 2022, 1216, 339991. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B. Zinc (II) and AIEgens: The “Clip Approach” for a Novel Fluorophore Family. A Review. Molecules 2021, 26, 4176. [Google Scholar] [CrossRef]
- Shustova, N.B.; McCarthy, B.D.; Dincă, M. Turn-On Fluorescence in Tetraphenylethylene-Based Metal–Organic Frameworks: An Alternative to Aggregation-Induced Emission. J. Am. Chem. Soc. 2011, 133, 20126–20129. [Google Scholar] [CrossRef]
- Neupane, L.N.; Hwang, G.W.; Lee, K.-H. Tuning of the selectivity of fluorescent peptidyl bioprobe using aggregation induced emission for heavy metal ions by buffering agents in 100% aqueous solutions. Biosens. Bioelectron. 2017, 92, 179–185. [Google Scholar] [CrossRef]
- Xie, Y.-Z.; Shan, G.-G.; Li, P.; Zhou, Z.-Y.; Su, Z.-M. A novel class of Zn (II) Schiff base complexes with aggregation-induced emission enhancement (AIEE) properties: Synthesis, characterization and photophysical/electrochemical properties. Dye. Pigment. 2013, 96, 467–474. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, X.; Li, N.; Xu, C.; Wu, R.; Zhu, B.; Zhang, G.; Bi, S.; Fan, Y. Zn-MOFs based luminescent sensors for selective and highly sensitive detection of Fe3+ and tetracycline antibiotic. J. Pharm. Biomed. Anal. 2020, 188, 113444. [Google Scholar] [CrossRef]
- Zhu, B.; Zong, Z.; Zhang, X.; Zhang, D.; Cui, L.; Bi, C.; Fan, Y. Highly Selective and Stable Zn (II)-Based Metal–Organic Frameworks for the Detections of Tetracycline Antibiotic and Acetone in Aqueous System. Appl. Organomet. Chem. 2020, 34, e5518. [Google Scholar] [CrossRef]
- Schwartz, M.A. Catalysis of hydrolysis and aminolysis of benzylpenicillin mediated by a ternary complex with zinc ion and Tris(hydroxymethyl)aminomethane. Bioorganic Chem. 1982, 11, 4–18. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, S.; Sun, S.; Yang, Y.; Zhang, Y.; Liu, J.; Dong, Y.; Su, H.; Tan, T. A molecular recognition assisted colorimetric aptasensor for tetracycline. RSC Adv. 2016, 6, 45645–45651. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.; Jia, L.; Zhu, T.; Chen, X.; Zhao, T. A smartphone-integrated method for visual detection of tetracycline. Chem. Eng. J. 2021, 416, 127741. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Y.; Yang, X. Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 2018, 10, 8139–8145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Chen, L. Preparation of multifunctional magnetic–fluorescent nanocomposites for analysis of tetracycline hydrochloride. New J. Chem. 2015, 39, 9976–9982. [Google Scholar] [CrossRef]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef] [PubMed]







| Person A | Person B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||
| morning | 1 | 51.0 | 43.0 | 39.1 | 46.1 | 49.3 | 48.2 | 36.8 | 39.1 | 56.3 | 43.7 |
| 2 | 46.8 | 37.6 | 42.7 | 45.6 | 56.0 | 47.2 | 45.2 | 40.5 | 43.5 | 42.7 | |
| 3 | 43.8 | 55.7 | 42.5 | 47.7 | 49.9 | 48.6 | 39.4 | 39.0 | 56.2 | 35.3 | |
| afternoon | 1 | 50.1 | 45.9 | 41.8 | 44.9 | 59.4 | 50.4 | 42.8 | 41.7 | 50.7 | 39.1 |
| 2 | 43.1 | 55.3 | 40.5 | 44.0 | 60.4 | 38.5 | 43.9 | 49.3 | 57.4 | 42.8 | |
| 3 | 41.7 | 47.6 | 40.2 | 44.3 | 51.7 | 41.8 | 44.5 | 42.3 | 55.5 | 43.2 | |
| mean (n = 6) | 46.1 | 47.5 | 41.1 | 45.5 | 54.5 | 45.8 | 42.1 | 42.0 | 53.3 | 41.1 | |
| SD | 3.5 | 6.4 | 1.3 | 1.2 | 4.4 | 4.2 | 3.0 | 3.5 | 4.9 | 3.0 | |
| RSD (%) | 7.7 | 13.5 | 3.1 | 2.7 | 8.1 | 9.2 | 7.2 | 8.3 | 9.1 | 7.4 | |
| Intermediate precision Person A (n = 30 for each person) | mean | 46.9 | Person B | mean | 44.9 | ||||||
| SD | 5.8 | SD | 5.9 | ||||||||
| RSD (%) | 12.4 | RSD (%) | 13.1 | ||||||||
| Precision (n = 60) | mean | 45.9 | |||||||||
| SD | 6.0 | Accuracy (n = 60) | 96.4% | ||||||||
| RSD (%) | 13.0 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.-C.; Tsai, J.-J.; Chen, Y.-C. Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline. Molecules 2022, 27, 8403. https://doi.org/10.3390/molecules27238403
Zhan Y-C, Tsai J-J, Chen Y-C. Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline. Molecules. 2022; 27(23):8403. https://doi.org/10.3390/molecules27238403
Chicago/Turabian StyleZhan, Yan-Cen, Jia-Jen Tsai, and Yu-Chie Chen. 2022. "Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline" Molecules 27, no. 23: 8403. https://doi.org/10.3390/molecules27238403
APA StyleZhan, Y. -C., Tsai, J. -J., & Chen, Y. -C. (2022). Zinc Ion-Based Switch-on Fluorescence-Sensing Probes for the Detection of Tetracycline. Molecules, 27(23), 8403. https://doi.org/10.3390/molecules27238403