Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ti2SnC MP Characterization
2.2. Characterization of Membranes
2.3. Performance of the Membranes
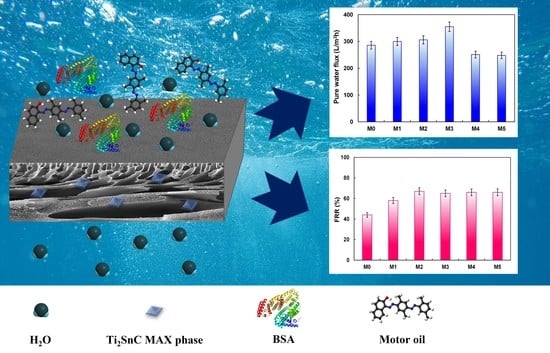
2.3.1. Permeability and Antifouling Features of the Membranes
2.3.2. Membrane Separation Ability
3. Experimental
3.1. Chemicals
3.2. Ti2SnC MP Synthesis
3.3. MP/PES Blended Membranes Fabrication
3.4. Characterization Techniques
3.5. Membranes’ Filtration Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- He, S.; Zhan, Y.; Hu, J.; Zhang, G.; Zhao, S.; Feng, Q.; Yang, W. Chemically stable two-dimensional MXene@ UIO-66-(COOH)2 composite lamellar membrane for multi-component pollutant-oil-water emulsion separation. Compos. B Eng. 2020, 197, 108188. [Google Scholar] [CrossRef]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, H.-Y.; Abdalkarim, S.Y.H.; Wang, C.; Tang, F.; Marek, J.; Chen, W.-L.; Militky, J.; Yao, J.-M. Green one-step synthesis of ZnO/cellulose nanocrystal hybrids with modulated morphologies and superfast absorption of cationic dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhan, Y.; Bai, Y.; Hu, J.; Li, Y.; Zhang, G.; Zhao, S. Gravity-driven and high flux super-hydrophobic/super-oleophilic poly(arylene ether nitrile) nanofibrous composite membranes for efficient water-in-oil emulsions separation in harsh environments. Compos. B Eng. 2019, 177, 107439. [Google Scholar] [CrossRef]
- Dhand, V.; Hong, S.K.; Li, L.; Kim, J.-M.; Kim, S.H.; Rhee, K.Y.; Lee, H.W. Fabrication of robust, ultrathin and light weight, hydrophilic, PVDF-CNT membrane composite for salt rejection. Compos. B Eng. 2019, 160, 632–643. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Song, Y.; Yan, J.; Wang, Y.; Ren, G. A waterproofing textile with robust superhydrophobicity in either air or oil surroundings. J. Taiwan Inst. Chem. Eng. 2017, 71, 421–425. [Google Scholar] [CrossRef]
- Abadikhah, H.; Naderi Kalali, E.; Khodi, S.; Xu, X.; Agathopoulos, S. Multifunctional thin-film nanofiltration membrane incorporated with reduced graphene oxide@ TiO2@ Ag nanocomposites for high desalination performance, dye retention, and antibacterial properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Dubey, N.C.; Stamm, M. Polyethylene glycol cross-linked sulfonated polyethersulfone based filtration membranes with improved antifouling tendency. J. Membr. Sci. 2014, 453, 263–274. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.-Y.; Lin, H.-J.; Huang, C.-C. Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling—A review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Ormanci-Acar, T.; Keskin, B.; Korkut, S.; Mutlu-Salmanlı, O.; Turken, T.; Koseoglu-Imer, D.Y.; Demir, T.U.; Menceloglu, Y.Z.; Unal, S.; Koyuncu, I. Fabrication of halloysite nanotubes embedded thin film nanocomposite membranes for dye removal. J. Appl. Polym. Sci. 2021, 138, 50986. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.H.; Lee, J.M. Graphene-based advanced membrane applications in organic solvent nanofiltration. Adv. Funct. Mater. 2021, 31, 2006949. [Google Scholar] [CrossRef]
- He, M.; Zhang, R.; Zhang, K.; Liu, Y.; Su, Y.; Jiang, Z. Reduced graphene oxide aerogel membranes fabricated through hydrogen bond mediation for highly efficient oil/water separation. J. Mater. Chem. A 2019, 7, 11468–11477. [Google Scholar] [CrossRef]
- Memon, F.H.; Rehman, F.; Lee, J.; Soomro, F.; Iqbal, M.; Khan, S.M.; Ali, A.; Thebo, K.H.; Choi, K.H. Transition metal dichalcogenide-based membranes for water desalination, gas separation, and energy storage. Sep. Purif. Rev. 2022, 1–15. [Google Scholar] [CrossRef]
- Donato, L.; Garofalo, A.; Drioli, E.; Alharbi, O.; Aljlil, S.; Criscuoli, A.; Algieri, C. Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep. Purif. Technol. 2020, 237, 116376. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Barati Farimani, A. Water desalination with two-dimensional metal–organic framework membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, X.; He, S.; Yang, Q.; He, Y. Design of durable and efficient poly(arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. J. Chem. Eng. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Haftani, M.; Heydari, M.S.; Baharvandi, H.R.; Ehsani, N. Studying the oxidation of Ti2AlC MAX phase in atmosphere: A review. Int. J. Refract. Hard. Met. 2016, 61, 51–60. [Google Scholar] [CrossRef]
- Lapauw, T.; Halim, J.; Lu, J.; Cabioc’h, T.; Hultman, L.; Barsoum, M.W.; Lambrinou, K.; Vleugels, J. Synthesis of the novel Zr3AlC2 MAX phase. J. Eur. Ceram. Soc. 2016, 36, 943–947. [Google Scholar] [CrossRef]
- Ansarian, Z.; Khataee, A.; Arefi-Oskoui, S.; Orooji, Y.; Lin, H. Ultrasound-assisted catalytic activation of peroxydisulfate on Ti3GeC2 MAX phase for efficient removal of hazardous pollutants. Mater. Today Chem. 2022, 24, 100818. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Ye, H.; Jin, W.; Cui, Z. Two-dimensional MXene incorporated chitosan mixed-matrix membranes for efficient solvent dehydration. J. Membr. Sci. 2018, 563, 625–632. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Azam, R.S.; Mahmoud, K.A. A fouling-resistant mixed-matrix nanofiltration membrane based on covalently cross-linked Ti3C2TX (MXene)/cellulose acetate. J. Membr. Sci. 2020, 607, 118139. [Google Scholar] [CrossRef]
- Sun, Z.; Music, D.; Ahuja, R.; Li, S.; Schneider, J.M. Bonding and classification of nanolayered ternary carbides. Phys. Rev. B 2004, 70, 092102. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Liu, L.; Cao, K.; Yang, D.; Gong, C.; Lei, H.; Hang, H.; Yao, W.; Xu, J. Intercalation and delamination of Ti2SnC with high lithium ion storage capacity. Nanoscale 2021, 13, 7355–7361. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hong, J.M. Effect of Nano-Sized ZnO Particle Addition on PVDF Ultrafiltration Membrane Performance. Adv. Mater. Res. 2011, 311, 1818–1821. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Behrouz, S.J.; Vatanpour, V.; Gharamaleki, S.H.; Orooji, Y.; Safarpour, M. Development of MoS2/O-MWCNTs/PES blended membrane for efficient removal of dyes, antibiotic, and protein. Sep. Purif. Technol. 2022, 280, 119822. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, S.; Li, M.; Xue, A.; Zhao, Y.; Peng, W.; Xing, W. PVDF mixed matrix ultrafiltration membrane incorporated with deformed rebar-like Fe3O4–palygorskite nanocomposites to enhance strength and antifouling properties. J. Membr. Sci. 2020, 612, 118467. [Google Scholar] [CrossRef]
- Robert, N.W. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Ma, B.; Yang, J.; Sun, Q.; Jakpa, W.; Hou, X.; Yang, Y. Influence of cellulose/[Bmim] Cl solution on the properties of fabricated NIPS PVDF membranes. J. Mater. Sci. 2017, 52, 9946–9957. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J. Membr. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Wan, Z.; Jiang, Y. Synthesis-structure-performance relationships of nanocomposite polymeric ultrafiltration membranes: A comparative study of two carbon nanofillers. J. Membr. Sci. 2021, 620, 118847. [Google Scholar] [CrossRef]
- Shakak, M.; Rezaee, R.; Maleki, A.; Jafari, A.; Safari, M.; Shahmoradi, B.; Daraei, H.; Lee, S.-M. Synthesis and characterization of nanocomposite ultrafiltration membrane (PSF/PVP/SiO2) and performance evaluation for the removal of amoxicillin from aqueous solutions. Environ. Technol. Innov. 2020, 17, 100529. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shokri, E.; Shahed, E.; Hermani, M.; Etemadi, H. Towards enhanced fouling resistance of PVC ultrafiltration membrane using modified montmorillonite with folic acid. Appl. Clay Sci. 2021, 200, 105906. [Google Scholar] [CrossRef]
- Kallem, P.; Othman, I.; Ouda, M.; Hasan, S.W.; AlNashef, I.; Banat, F. Polyethersulfone hybrid ultrafiltration membranes fabricated with polydopamine modified ZnFe2O4 nanocomposites: Applications in humic acid removal and oil/water emulsion separation. Process Saf. Environ. Prot. 2021, 148, 813–824. [Google Scholar] [CrossRef]
- Amid, M.; Nabian, N.; Delavar, M. Fabrication of polycarbonate ultrafiltration mixed matrix membranes including modified halloysite nanotubes and graphene oxide nanosheets for olive oil/water emulsion separation. Sep. Purif. Technol. 2020, 251, 117332. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. J. Chem. Eng. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing antifouling capability of PES membrane via mixing with various types of polymer modified multi-walled carbon nanotube. J. Membr. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Okoye, P.U.; Otitoju, T.A.; Ariffin, K.S. Aragonite precipitated calcium carbonate from magnesium rich carbonate rock for polyethersulfone hollow fibre membrane application. J. Clean. Prod. 2018, 195, 79–92. [Google Scholar] [CrossRef]
- Lai, G.; Yusob, M.; Lau, W.; Gohari, R.J.; Emadzadeh, D.; Ismail, A.; Goh, P.; Isloor, A.; Arzhandi, M.R.-D. Novel mixed matrix membranes incorporated with dual-nanofillers for enhanced oil-water separation. Sep. Purif. Technol. 2017, 178, 113–121. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Fouling resistant mixed matrix polyethersulfone membranes blended with magnetic nanoparticles: Study of magnetic field induced casting. Sep. Purif. Technol. 2013, 109, 111–121. [Google Scholar] [CrossRef]
- Li, S.-B.; Bei, G.-P.; Zhai, H.-X.; Zhou, Y.; Li, C.-W. Synthesis of Ti2SnC at low-temperature using mechanically activated sintering process. Mater. Sci. Eng. A 2007, 457, 282–286. [Google Scholar] [CrossRef]
- Li, S.-B.; Bei, G.-P.; Zhai, H.-X.; Zhou, Y. Synthesis of Ti2SnC from Ti/Sn/TiC powder mixtures by pressureless sintering technique. Mater. Lett. 2006, 60, 3530–3532. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Vatanpour, V. Modification of polyethersulfone ultrafiltration membrane using ultrasonic-assisted functionalized MoS2 for treatment of oil refinery wastewater. Sep. Purif. Technol. 2020, 238, 116495. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]






| Membrane | Porosity (%) | Mean Pore Size (nm) | Contact Angle (°) | Roughness Parameters | ||
|---|---|---|---|---|---|---|
| Sa (nm) | Sq (nm) | Sy (nm) | ||||
| M0 | 77.6 ± 1.4 | 9.27 ± 0.97 | 63.3 ± 2.9 | 6.29 | 8.12 | 40.02 |
| M1 | 79.7 ± 1.6 | 9.71 ± 0.19 | 54.1 ± 6.7 | 7.32 | 8.95 | 47.30 |
| M2 | 83.1 ± 1.3 | 9.72 ± 0.33 | 50.7 ± 2.5 | 8.70 | 11.65 | 100.01 |
| M3 | 85.2 ± 5.6 | 10.14 ± 0.69 | 49.7 ± 4.6 | 8.82 | 10.80 | 62.92 |
| M4 | 84.3 ± 2.9 | 8.41 ± 0.30 | 51.5 ± 1.8 | 8.43 | 10.56 | 46.16 |
| M5 | 79.6 ± 3.5 | 8.15 ± 0.80 | 53.2 ± 1.9 | 8.17 | 11.46 | 55.35 |
| Membrane | Nano-Additive | Pore Former | Operational Pressure (MPa) | Pure Water Flux (L/m2h) | FRR (%) | Ref. |
|---|---|---|---|---|---|---|
| PES (20 wt.%) | GO (0.3 wt.%) | T904 (5 wt.%) | 0.1 | 245 | 62 | [43] |
| PES (20 wt.%) | MWCNT (0.1 wt.%) | PVP (1 wt.%) | 0.5 | 23 | 95 | [44] |
| PES (17.25 wt.%) | A-PCC (3 wt.%) | PEG (1.75 wt.%) | 0.15 | 180 | 86.4 | [45] |
| PES (11.54 wt.%) | HMO (0.75)–TiO2 (0.25) | PVP (1.15 wt.%) | 0.1 | 28.48 | 91.5 | [46] |
| PES (18 wt.%) | PANI/Fe3O4 (0.1 wt.%) | PVP (1 wt.%) | 0.4 | 52 | 80 | [47] |
| PES (18 wt.%) | Ti2SnC MAX phase (0.5 wt.%) | PVP (1 wt.%) | 0.3 | 355 | 65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarpour, M.; Hosseinpour, S.; Haddad Irani-nezhad, M.; Orooji, Y.; Khataee, A. Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation. Molecules 2022, 27, 8914. https://doi.org/10.3390/molecules27248914
Safarpour M, Hosseinpour S, Haddad Irani-nezhad M, Orooji Y, Khataee A. Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation. Molecules. 2022; 27(24):8914. https://doi.org/10.3390/molecules27248914
Chicago/Turabian StyleSafarpour, Mahdie, Shahla Hosseinpour, Mahsa Haddad Irani-nezhad, Yasin Orooji, and Alireza Khataee. 2022. "Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation" Molecules 27, no. 24: 8914. https://doi.org/10.3390/molecules27248914
APA StyleSafarpour, M., Hosseinpour, S., Haddad Irani-nezhad, M., Orooji, Y., & Khataee, A. (2022). Fabrication of Ti2SnC-MAX Phase Blended PES Membranes with Improved Hydrophilicity and Antifouling Properties for Oil/Water Separation. Molecules, 27(24), 8914. https://doi.org/10.3390/molecules27248914