High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired
Abstract
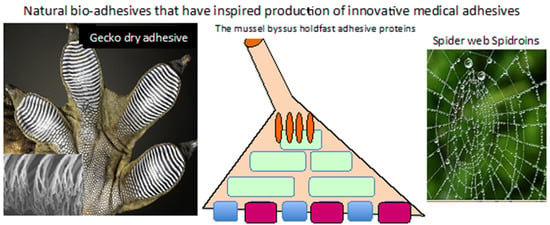
:1. Introduction
2. Mussel Foot Proteins and the Adhesive Process
3. Barnacle Cement
4. Sandcastle Worm, Caddis-Fly Fly Larvae Cement
5. Slug Adhesive
6. Gecko Dry Adhesive
7. Spider Silk
7.1. Emerging New Spider Web Technologies
7.2. Spider Webs Are Electroconductive and Harvest Atmospheric Moisture
7.3. Spider Web Silk a Putative Polymer Facilitating Novel Developments in the Design of Biomedical, Micro- and Nano-Electronic Devices and Biosensors
7.4. High Precision Air Filters Based on Spider Proteins
8. Caterpillar Silk
The Silk Produced by Silk-Worm Larvae Bombyx Mori
9. Medical Adhesives
10. Future Research with Bioadhesives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Financial Disclosure
References
- Zhao, H.; Sun, C.; Stewart, R.J.; Waite, J.H. Cement Proteins of the Tube-building Polychaete Phragmatopoma californica. J. Biol. Chem. 2005, 280, 42938–42944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Ren, S.; Wei, S.; Sun, C.; Zhong, C. Natural and bio-inspired underwater adhesives: Current progress and new perspectives. APL Mater. 2017, 5, 116102. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.; Glattauer, V.; Peng, Y.Y.; Vaughan, P.R.; Werkmeister, J.A.; Tyler, M.H.J.; Ramshaw, J.A.M. An Adhesive Secreted by Australian Frogs of the Genus Notaden. In Biological Adhesives; Smith, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 223–245. [Google Scholar]
- Graham, L.D.; Glattauer, V.; Huson, M.G.; Maxwell, J.M.; Knott, R.B.; White, J.W.; Vaughan, P.R.; Peng, Y.; Tyler, M.J.; Werkmeister, J.A.; et al. Characterization of a Protein-based Adhesive Elastomer Secreted by the Australian Frog Notaden bennetti. Biomacromolecules 2005, 6, 3300–3312. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.; Ramshaw, J.A. An Adhesive Derived from Amphibian Skin Secretions. WO2002022756A1, 21 March 2002. [Google Scholar]
- Noel, A.C.; Guo, H.-Y.; Mandica, M.; Hu, D.L. Frogs use a viscoelastic tongue and non-Newtonian saliva to catch prey. J. R. Soc. Interface 2017, 14, 20160764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, A.; Hu, D. The tongue as a gripper. J. Exp. Biol. 2018, 221 Pt 7, jeb176289. [Google Scholar] [CrossRef] [Green Version]
- Fowler, J.E.; Kleinteich, T.; Franz, J.; Jaye, C.; Fischer, D.A.; Gorb, S.N.; Weidner, T.; Baio, J.E. Surface chemistry of the frog sticky-tongue mechanism. Biointerphases 2018, 13, 06E408. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ueyama, D.; Kobayashi, R. The advantage of mucus for adhesive locomotion in gastropods. J. Theor. Biol. 2014, 353, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Kleinteich, T.; Gorb, S.N. Frog tongue acts as muscle-powered adhesive tape. R. Soc. Open Sci. 2015, 2, 150333. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.H.; del Alamo, J.C.; Rodríguez-Rodríguez, J.; Lasheras, J.C. The mechanics of the adhesive locomotion of terrestrial gastropods. J. Exp. Biol. 2010, 213, 3920–3933. [Google Scholar] [CrossRef] [Green Version]
- Newar, J.; Ghatak, A. Studies on the Adhesive Property of Snail Adhesive Mucus. Langmuir 2015, 31, 12155–12160. [Google Scholar] [CrossRef]
- Wilks, A.; Rabice, S.R.; Garbacz, H.S.; Harro, C.C.; Smith, A.M. Double-network gels and the toughness of terrestrial slug glue. J. Exp. Biol. 2015, 218 Pt 19, 3128–3137. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.D.; Danon, S.J.; Johnson, G.; Braybrook, C.; Hart, N.K.; Varley, R.J.; Evans, M.D.M.; McFarland, G.A.; Tyler, M.J.; Werkmeister, J.A.; et al. Biocompatibility and modification of the protein-based adhesive secreted by the Australian frogNotaden bennetti. J. Biomed. Mater. Res. Part A 2010, 93, 429–441. [Google Scholar] [CrossRef]
- Graham, L.D.; Glattauer, V.; Li, D.; Tyler, M.J.; Ramshaw, J.A. The adhesive skin exudate of Notaden bennetti frogs (Anura: Limnodynastidae) has similarities to the prey capture glue of Euperipatoides sp. velvet worms (Onychophora: Peripatopsidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 165, 250–259. [Google Scholar] [CrossRef]
- Graham, L. Biological Adhesives from Nature. In Encyclopedia of Biomaterials and Biomedical Engineering, 2nd ed.; Wnek, G., Bowlin, G.L., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Boca Raton, FL, USA, 2008; Volume 1, pp. 236–254. [Google Scholar]
- Millar, N.L.; Bradley, T.A.; Walsh, N.A.; Appleyard, R.C.; Tyler, M.J.; Murrell, G.A. Frog glue enhances rotator cuff repair in a laboratory cadaveric model. J. Shoulder Elb. Surg. 2009, 18, 639–645. [Google Scholar] [CrossRef]
- Szomor, Z.L.; Murrell, G.A.C.; Appleyard, R.C.; Tyler, M.J. Meniscal repair with a new biological glue: An ex vivo study. Tech. Knee Surg. 2009, 7, 261–265. [Google Scholar] [CrossRef]
- Tyler, M. Adhesive dermal secretions of the Amphibia, with particular reference to the Australian Limnodynastid genus Notaden. In Biological Adhesive Systems. From Nature to Technical and Medical Application; von Byern, J.G., Ed.; Springer: Wien, Austria; New York, NY, USA, 2010; pp. 181–186. [Google Scholar]
- Gould, J.; Valdez, J.W.; Upton, R. Adhesive defence mucus secretions in the red triangle slug (Triboniophorus graeffei) can incapacitate adult frogs. Ethology 2019, 125, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Karp, J.M. A Slick and Stretchable Surgical Adhesive. N. Engl. J. Med. 2017, 377, 2092–2094. [Google Scholar] [CrossRef]
- Majumder, A.; Ghatak, A.; Sharma, A. Microfluidic Adhesion Induced by Subsurface Microstructures. Science 2007, 318, 258–261. [Google Scholar] [CrossRef] [Green Version]
- Mengüç, Y.; Röhrig, M.; Abusomwan, U.; Hoelscher, H.; Sitti, M. Staying sticky: Contact self-cleaning of gecko-inspired adhesives. J. R. Soc. Interface 2014, 11, 20131205. [Google Scholar] [CrossRef] [Green Version]
- Persson, B. Wet adhesion with application to tree frog adhesive toe pads and tires. J. Phys. Condens. Matter 2007, 19, 376110. [Google Scholar] [CrossRef]
- Russell, A.P.; Stark, A.Y.; Higham, T.E. The Integrative Biology of Gecko Adhesion: Historical Review, Current Understanding, and Grand Challenges. Integr. Comp. Biol. 2019, 59, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Xia, Z.; Dai, L. Advanced gecko-foot-mimetic dry adhesives based on carbon nanotubes. Nanoscale 2013, 5, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, E.W.; Eason, E.V.; Christensen, D.L.; Cutkosky, M.R. Human climbing with efficiently scaled gecko-inspired dry adhesives. J. R. Soc. Interface 2015, 12, 20140675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sameoto, D.; Menon, C. Recent advances in the fabrication and adhesion testing of biomimetic dry adhesives. Smart Mater. Struct. 2010, 19, 103001. [Google Scholar] [CrossRef]
- Autumn, K.; Dittmore, A.; Santos, D.; Spenko, M.; Cutkosky, M. Frictional adhesion: A new angle on gecko attachment. J. Exp. Biol. 2006, 209 Pt 18, 3569–3579. [Google Scholar] [CrossRef] [Green Version]
- DeVries, J.G.; Scharer, B.M.; Romdenne, T.A. Ankle Stabilization With Arthroscopic Versus Open With Suture Tape Augmentation Techniques. J. Foot Ankle Surg. 2019, 58, 57–61. [Google Scholar] [CrossRef]
- Golden, T.; Friedman, A.M.; Jazayeri, R.; Sanderson, B.; Levy, E. Primary Repair of the Medial Collateral Ligament with a Double Row Suture Technique and Suture Tape Augmentation for Acute Tibial-Sided Injuries. Arthrosc. Tech. 2019, 8, e395–e398. [Google Scholar] [CrossRef] [Green Version]
- Heusdens, C.H.W.; Hopper, G.P.; Dossche, L.; Roelant, E.; Mackay, G.M. Anterior cruciate ligament repair with Independent Suture Tape Reinforcement: A case series with 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 60–67. [Google Scholar] [CrossRef]
- Monaco, E.; Mazza, D.; Redler, A.; Drogo, P.; Wolf, M.R.; Ferretti, A. Anterolateral Ligament Repair Augmented with Suture Tape in Acute Anterior Cruciate Ligament Reconstruction. Arthrosc. Tech. 2019, 8, e369–e373. [Google Scholar] [CrossRef]
- Seo, J.-B.; Heo, K.; Kim, S.-J.; Jung, J.-U.; Yoo, J.-S. Arthroscopic Acromioclavicular Fixation with Suture Tape Augmentation after Coracoclavicular Fixation With Dog Bone Button: Surgical Technique. Arthrosc. Tech. 2018, 7, e1197–e1203. [Google Scholar] [CrossRef] [Green Version]
- Aboalata, M.; Halawa, A.; Basyoni, Y. The Double Bankart Bridge: A Technique for Restoration of the Labral Footprint in Arthroscopic Shoulder Instability Repair. Arthrosc. Tech. 2017, 6, e43–e47. [Google Scholar] [CrossRef] [Green Version]
- Chapple, C.R.; Cruz, F.; Deffieux, X.; Milani, A.L.; Arlandis, S.; Artibani, W.; Bauer, R.M.; Burkhard, F.; Cardozo, L.; Castro-Diaz, D.; et al. Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence. Eur. Urol. 2017, 72, 424–431. [Google Scholar] [CrossRef]
- Filoni, A.; Bonamonte, D.; Vestita, M. An inexpensive wound closure strip. J. Am. Acad. Dermatol. 2016, 75, e29–e30. [Google Scholar] [CrossRef]
- Kitshoff, A.M.; Louwagie, J.; Or, M.; Devriendt, N.; Dehuisser, V.; Koenraadt, A.; Vandenabeele, S.; Sys, S.U.; De Rooster, H. Biomechanical properties of celiotomy wounds closed with tape and cyanoacrylate versus intradermal sutures. Veter- Surg. 2018, 47, 1087–1093. [Google Scholar] [CrossRef]
- Petros, P.; Abendstein, B. The mechanics of urethral closure, incontinence, and midurethral sling repair Part 3 surgical applications (1990–2016). Neurourol. Urodynamics 2019, 38, 818–824. [Google Scholar] [CrossRef]
- Van De Kar, A.L.; Koolbergen, D.R.; Van Avendonk, J.P.H.; Van Der Horst, C.M.A.M. Comparison of wound closure techniques in median sternotomy scars in children: Subcuticular suture versus Steri-Strip™ S. J. Plast. Surg. Hand Surg. 2019, 53, 161–166. [Google Scholar] [CrossRef]
- Yonguc, T.; Bozkurt, I.H.; Sen, V.; Aydogdu, O.; Yonguc, G.N.; Gunlusoy, B. Double-sling procedure for the surgical management of stress urinary incontinence with concomitant anterior vaginal wall prolapse. Int. Urol. Nephrol. 2015, 47, 1611–1617. [Google Scholar] [CrossRef]
- Crispim, J.F.; Fu, S.C.; Lee, Y.W.; Fernandes, H.A.; Jonkheijm, P.; Yung, P.S.; Saris, D.B. Bioactive Tape With BMP-2 Binding Peptides Captures Endogenous Growth Factors and Accelerates Healing After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2018, 46, 2905–2914. [Google Scholar] [CrossRef]
- Su, I.; Qin, Z.; Saraceno, T.; Krell, A.; Mühlethaler, R.; Bisshop, A.; Buehler, M.J. Imaging and analysis of a three-dimensional spider web architecture. J. R. Soc. Interface 2018, 15, 20180193. [Google Scholar] [CrossRef]
- Cranford, S.W.; Tarakanova, A.; Pugno, N.M.; Buehler, M.J. Nonlinear material behaviour of spider silk yields robust webs. Nature 2012, 482, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DAS, R.; Kumar, A.; Patel, A.; Vijay, S.; Saurabh, S.; Kumar, N. Biomechanical characterization of spider webs. J. Mech. Behav. Biomed. Mater. 2017, 67, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Behrooz, M.; Gordaninejad, F. A bioinspired adaptive spider web. Bioinspiration Biomim. 2017, 12, 16012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K. The Design Characteristics of Nature-inspired Buildings. Civ. Eng. Arch. 2018, 6, 88–107. [Google Scholar] [CrossRef] [Green Version]
- Lombardini, D.; Geyer, S. Cable Erection of Krasnodar Stadium Suspended Roof. Procedia Eng. 2016, 155, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Nicklisch, S.C.T.; Das, S.; Rodriguez, N.R.M.; Waite, J.H.; Israelachvili, J.N. Antioxidant efficacy and adhesion rescue by a recombinant mussel foot protein-6. Biotechnol. Prog. 2013, 29, 1587–1593. [Google Scholar] [CrossRef] [Green Version]
- Danner, E.W.; Kan, Y.; Hammer, M.U.; Israelachvili, J.N.; Waite, J.H. Adhesion of Mussel Foot Protein Mefp-5 to Mica: An Underwater Superglue. Biochemistry 2012, 51, 6511–6518. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the Adhesive Pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Linking Adhesive and Structural Proteins in the Attachment Plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150–26158. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.P.; Messersmith, P.; Israelachvili, J.; Waite, J. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Lu, Q.; Danner, E.; Waite, J.H.; Israelachvili, J.N.; Zeng, H.; Hwang, D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 2013, 10, 20120759. [Google Scholar] [CrossRef]
- Priemel, T.; Palia, R.; Babych, M.; Thibodeaux, C.J.; Bourgault, S.; Harrington, M.J. Compartmentalized processing of catechols during mussel byssus fabrication determines the destiny of DOPA. Proc. Natl. Acad. Sci. USA 2020, 117, 7613–7621. [Google Scholar] [CrossRef]
- Priemel, T.; Palia, G.; Förste, F.; Jehle, F.; Sviben, S.; Mantouvalou, I.; Zaslansky, P.; Bertinetti, L.; Harrington, M.J. Microfluidic-like fabrication of metal ion–cured bioadhesives by mussels. Science 2021, 374, 206–211. [Google Scholar] [CrossRef]
- Qin, C.-L.; Pan, Q.-D.; Qi, Q.; Fan, M.-H.; Sun, J.-J.; Li, N.-N.; Liao, Z. In-depth proteomic analysis of the byssus from marine mussel Mytilus coruscus. J. Proteom. 2016, 144, 87–98. [Google Scholar] [CrossRef]
- Waite, J.; Qin, X.X.; Coyne, K.J. The peculiar collagens of mussel byssus. Matrix Biol 1998, 17, 93–106. [Google Scholar] [CrossRef]
- Areyano, M.; Valois, E.; Carvajal, I.S.; Rajkovic, I.; Wonderly, W.R.; Kossa, A.; McMeeking, R.M.; Waite, J.H. Viscoelastic analysis of mussel threads reveals energy dissipative mechanisms. J. R. Soc. Interface 2022, 19, 20210828. [Google Scholar] [CrossRef]
- Waite, J.H.; Vaccaro, E.; Sun, C.; Lucas, J.M. Elastomeric gradients: A hedge against stress concentration in marine holdfasts? Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Coyne, K.; Qin, X.X.; Waite, J.H. Extensible collagen in mussel byssus: A natural block copolymer. Science 1997, 277, 1830–1832. [Google Scholar] [CrossRef]
- Qin, X.; Waite, J.H. Exotic collagen gradients in the byssus of the mussel Mytilus edulis. J. Exp. Biol. 1995, 198 Pt 3, 633–644. [Google Scholar] [CrossRef]
- Qin, X.; Coyne, K.J.; Waite, J.H. Tough tendons. Mussel byssus has collagen with silk-like domains. J. Biol. Chem. 1997, 272, 32623–32627. [Google Scholar] [CrossRef]
- Tunn, I.; Harrington, M.J.; Blank, K.G. Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels. Biomimetics 2019, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechel, S.; Hager, M.D.; Priemel, T.; Harrington, M.J. Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination. Biomimetics 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, M.; Elsner, M.B.; Scheibel, T. Lipid-Specific β-Sheet Formation in a Mussel Byssus Protein Domain. Biomacromolecules 2013, 14, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-X.; Waite, J.H. A potential mediator of collagenous block copolymer gradients in mussel byssal threads. Proc. Natl. Acad. Sci. USA 1998, 95, 10517–10522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Robertson, N.B.; Jewhurst, S.A.; Waite, J.H. Probing the Adhesive Footprints of Mytilus californianus Byssus. J. Biol. Chem. 2006, 281, 11090–11096. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Sun, C.; Jiang, F.; Yang, B.; Li, J.; Zhong, C.; Zheng, L.; Ding, H. Lipids as integral components in mussel adhesion. Soft Matter 2018, 14, 7145–7154. [Google Scholar] [CrossRef]
- Almeida, M.; Reis, R.L.; Silva, T.H. Marine invertebrates are a source of bioadhesives with biomimetic interest. Mater. Sci. Eng. C 2020, 108, 110467. [Google Scholar] [CrossRef]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-inspired bioadhesives in healthcare: Design parameters, current trends, and future perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef]
- Qiu, L.; See, A.A.Q.; Steele, T.W.J.; King, N.K.K. Bioadhesives in neurosurgery: A review. J. Neurosurg. 2019, 133, 1928–1938. [Google Scholar] [CrossRef]
- Shokri, M.; Dalili, F.; Kharaziha, M.; Baghaban Eslaminejad, M.; Ahmadi Tafti, H. Strong and bioactive bioinspired bio-materials, next generation of bone adhesives. Adv. Colloid Interface Sci. 2022, 305, 102706. [Google Scholar] [CrossRef]
- Yan, H.; Li, L.; Wang, Z.; Wang, Y.; Guo, M.; Shi, X.C.; Yeh, J.-M.; Zhang, P. Mussel-Inspired Conducting Copolymer with Aniline Tetramer as Intelligent Biological Adhesive for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 634–646. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef]
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Zhang, F.; Hu, W.; Gao, Z.; Zhang, Y.; Ding, M.; Liang, Q. Mussel Inspired Trigger-Detachable Adhesive Hydrogel. Small 2022, 18, 2200336. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Gerhard, E.M.; Zhang, S.; Rodríguez, F.I.P.; Pan, T.; Yang, H.; Lin, Y.; Yang, J.; Cheng, H. Smart bioadhesives for wound healing and closure. Bioact. Mater. 2022, 19, 360–375. [Google Scholar] [CrossRef]
- Liang, C.; Strickland, J.; Ye, Z.; Wu, W.; Hu, B.; Rittschof, D. Biochemistry of Barnacle Adhesion: An Updated Review. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-C.; Wong, Y.H.; Sung, C.-H.; Chan, B.K.K. Histology and transcriptomic analyses of barnacles with different base materials and habitats shed lights on the duplication and chemical diversification of barnacle cement proteins. BMC Genom. 2021, 22, 783. [Google Scholar] [CrossRef]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6, 36219. [Google Scholar] [CrossRef]
- Tilbury, M.; McCarthy, S.; Domagalska, M.; Ederth, T.; Power, A.M.; Wall, J.G. The expression and characterization of recom-binant cp19k barnacle cement protein from Pollicipes pollicipes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190205. [Google Scholar] [CrossRef]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef] [PubMed]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5, 1700762. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.; Liang, C.; Bi, X.; Wu, J.; Ye, Z.; Wu, W.; Hu, B. Adhesive Materials Inspired by Barnacle Underwater Adhesion: Biological Principles and Biomimetic Designs. Front. Bioeng. Biotechnol. 2022, 10, 870445. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, M.; Rizzo, G.; Farinola, G.M.; Omenetto, F.G. Bioinspired Biomaterial Composite for All-Water-Based High-Performance Adhesives. Adv. Sci. 2021, 8, e2004786. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Antas, P.; Castro, L.F.C.; Campos, A.; Vasconcelos, V.; Pereira, F.; Cunha, I. Comparative Analysis of the Adhesive Proteins of the Adult Stalked Goose Barnacle Pollicipes pollicipes (Cirripedia: Pedunculata). Mar. Biotechnol. 2019, 21, 38–51. [Google Scholar] [CrossRef]
- Schultzhaus, J.; Hervey, W.J.; Taitt, C.R.; So, C.R.; Leary, D.H.; Wahl, K.J.; Spillmann, C.M. Comparative analysis of stalked and acorn barnacle adhesive proteomes. Open Biol. 2021, 11, 210142. [Google Scholar] [CrossRef]
- Hennebert, E.; Maldonado, B.; Ladurner, P.; Flammang, P.; Santos, R. Experimental strategies for the identification and char-acterization of adhesive proteins in animals: A review. Interface Focus 2015, 5, 20140064. [Google Scholar] [CrossRef] [Green Version]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Lee, Y.J.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5, 4414. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wu, Y.; Wang, L.; Zhang, M.; Chen, X.; Liu, M.; Fan, J.; Liu, J.; Zhou, F.; Wang, Z. Bio-inspired reversible underwater adhesive. Nat. Commun. 2017, 8, 2218. [Google Scholar] [CrossRef]
- Power, A.M.; Klepal, W.; Zheden, V.; Jonker, J.; McEvilly, P.; von Byern, J. Mechanisms of Adhesion in Adult Barnacles. In Biological Adhesive Systems; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria, 2010; pp. 153–168. [Google Scholar] [CrossRef]
- Essock-Burns, T.; Gohad, N.V.; Orihuela, B.; Mount, A.S.; Spillmann, C.M.; Wahl, K.J.; Rittschof, D. Barnacle biology before, during and after settlement and metamorphosis: A study of the interface. J. Exp. Biol. 2017, 220 Pt 2, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.; Bonaventura, J.; Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212 Pt 2, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Jonker, J.-L.; Morrison, L.; Lynch, E.P.; Grunwald, I.; von Byern, J.; Power, A.M. The chemistry of stalked barnacle adhesive (Lepas anatifera). Interface Focus 2015, 5, 20140062. [Google Scholar] [CrossRef] [Green Version]
- Davidson, I.; Scianni, C.; Hewitt, C.; Everett, R.; Holm, E.; Tamburri, M.; Ruiz, G. Mini-review: Assessing the drivers of ship biofouling management—Aligning industry and biosecurity goals. Biofouling 2016, 32, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Townsin, R.L. The Ship Hull Fouling Penalty. Biofouling 2003, 19, 9–15. [Google Scholar] [CrossRef]
- Holm, E.R. Barnacles and Biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liang, C.; Zhang, X.; Li, J.; Huang, J.; Zeng, L.; Ye, Z.; Hu, B.; Wu, W. Amyloid fibril aggregation: An insight into the underwater adhesion of barnacle cement. Biochem. Biophys. Res. Commun. 2017, 493, 654–659. [Google Scholar] [CrossRef]
- Nakano, M.; Kamino, K. Amyloid-like Conformation and Interaction for the Self-Assembly in Barnacle Underwater Cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef]
- So, C.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.; North, S.; Oh, C.S.; et al. Oxidase Activity of the Barnacle Adhesive Interface Involves Perox-ide-Dependent Catechol Oxidase and Lysyl Oxidase Enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S. Adaptation of Caddisfly Larval Silks to Aquatic Habitats by Phosphorylation of H-Fibroin Serines. Biomacromolecules 2010, 11, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, N.; Sehnal, F.; Mita, A.K.; Tamura, T. Protein Composition of Silk Filaments Spun under Water by Caddisfly Larvae. Biomacromolecules 2006, 7, 3370–3378. [Google Scholar] [CrossRef] [PubMed]
- Kronenberger, K.; Vollrath, F.; Moore, P.G.; Halcrow, K. Spinning a Marine Silk for the Purpose of Tube-Building. J. Crustac. Biol. 2012, 32, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.J.; Weaver, J.C.; Morse, D.E.; Waite, J.H. The tube cement of Phragmatopoma californica: A solid foam. J. Exp. Biol. 2004, 207 Pt 26, 4727–4734. [Google Scholar] [CrossRef] [Green Version]
- Endrizzi, B.J.; Stewart, R.J. Glueomics: An Expression Survey of the Adhesive Gland of the Sandcastle Worm. J. Adhes. 2009, 85, 546–559. [Google Scholar] [CrossRef]
- Stewart, R.J.; Ransom, T.C.; Hlady, V. Natural underwater adhesives. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Sanai, K.; Nakagaki, M. A Novel Bioadhesive Protein of Silk Filaments Spun Underwater by Caddisfly Larvae. Adv. Mater. Res. 2009, 79–82, 1631–1634. [Google Scholar] [CrossRef]
- Wang, C.-S.; Pan, H.; Weerasekare, G.M.; Stewart, R.J. Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk. J. R. Soc. Interface 2015, 12, 20150710. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Chen, Z.-Q.; Lu, Z.; Li, J.; Hu, W.; Li, S.; Xu, Z. Exceptionally preserved caddisfly larval cases (Insecta) from the lower Cretaceous of the Liupanshan basin, Western China. J. Earth Sci. 2015, 26, 192–202. [Google Scholar] [CrossRef]
- Smith, A.; Robinson, T.; Salt, M.; Hamilton, K.; Silvia, B.; Blasiak, R. Robust cross-links in molluscan adhesive gels: Testing for contributions from hydrophobic and electrostatic interactions. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 152, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Werneke, S.W.; Swann, C.; Farquharson, L.A.; Hamilton, K.S.; Smith, A.M. The role of metals in molluscan adhesive gels. J. Exp. Biol. 2007, 210 Pt 12, 2137–2145. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Park, J.; Bhatta, R.; Liu, Y.; Bo, Y.; Zhou, J.; Wang, H. A double crosslinking adhesion mechanism for developing tough hydrogel adhesives. Acta Biomater. 2022, 150, 199–210. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Slug-inspired glue stays sticky when wet. Nature 2017, 548, 9. [Google Scholar] [CrossRef]
- Lang, N.; Pereira, M.J.; Lee, Y.; Friehs, I.; Vasilyev, N.V.; Feins, E.N.; Ablasser, K.; O’Cearbhaill, E.D.; Xu, C.; Fabozzo, A.; et al. A Blood-Resistant Surgical Glue for Minimally Invasive Repair of Vessels and Heart Defects. Sci. Transl. Med. 2014, 6, 218ra6. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Song, H.W.; Park, H.J.; Kwak, M.K. Surface Adaptable and Adhesion Controllable Dry Adhesive with Shape Memory Polymer. Macromol. Rapid Commun. 2022, 43, 2200012. [Google Scholar] [CrossRef]
- Dayan, C.B.; Chun, S.; Krishna-Subbaiah, N.; Drotlef, D.; Akolpoglu, M.B.; Sitti, M. 3D Printing of Elastomeric Bioinspired Complex Adhesive Microstructures. Adv. Mater. 2021, 33, 2103826. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, D.; Meng, F.; Yang, B.; Shi, Z.; Wang, X.; Li, Q.; Nie, C.; Liu, S.; Xue, L. Adhesion Enhancement of Micropillar Array by Combining the Adhesive Design from Gecko and Tree Frog. Small 2021, 17, e2005493. [Google Scholar] [CrossRef]
- Son, D.; Liimatainen, V.; Sitti, M. Machine Learning-Based and Experimentally Validated Optimal Adhesive Fibril Designs. Small 2021, 17, 2102867. [Google Scholar] [CrossRef]
- Stark, A.Y.; Mitchell, C.T. Stick or Slip: Adhesive Performance of Geckos and Gecko-Inspired Synthetics in Wet Environments. Integr. Comp. Biol. 2019, 59, 214–226. [Google Scholar] [CrossRef]
- Boogaart, L.M.v.D.; Langowski, J.K.A.; Amador, G.J. Studying Stickiness: Methods, Trade-Offs, and Perspectives in Measuring Reversible Biological Adhesion and Friction. Biomimetics 2022, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion advances: From nanomaterials to biomimetic adhesion and applications. Soft Matter 2022, 18, 3447–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mcadams, D.A., II; Grunlan, J.C. Nano/Micro-Manufacturing of Bioinspired Materials: A Review of Methods to Mimic Natural Structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Review: Mapping proteins localized in adhesive setae of the tokay gecko and their possible influence on the mechanism of adhesion. Protoplasma 2018, 255, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Immunolocalization of corneous proteins including a serine-tyrosine-rich beta-protein in the adhesive pads in the tokay gecko. Microsc. Res. Tech. 2020, 83, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.H.; Holler, K.R.; Baio, J.E.; Jaye, C.; Fischer, D.A.; Gorb, S.N.; Weidner, T. Evidence that gecko setae are coated with an ordered nanometre-thin lipid film. Biol. Lett. 2022, 18, 20220093. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Stark, A.Y.; Niewiarowski, P.H.; Miyoshi, T.; Dhinojwala, A. NMR spectroscopy reveals the presence and association of lipids and keratin in adhesive gecko setae. Sci. Rep. 2015, 5, 9594. [Google Scholar] [CrossRef] [Green Version]
- Holler, K.R.; Rasmussen, M.A.; Baio, J.E.; Jaye, C.; Fischer, D.A.; Gorb, S.N.; Weidner, T. Structure of Keratins in Adhesive Gecko Setae Determined by Near-Edge X-ray Absorption Fine Structure Spectromicroscopy. J. Phys. Chem. Lett. 2022, 13, 2193–2196. [Google Scholar] [CrossRef]
- Römer, L.; Scheibel, T. The elaborate structure of spider silk: Structure and function of a natural high performance fiber. Prion 2008, 2, 154–161. [Google Scholar] [CrossRef]
- Sahni, V.; Labhasetwar, D.V.; Dhinojwala, A. Spider Silk Inspired Functional Microthreads. Langmuir 2012, 28, 2206–2210. [Google Scholar] [CrossRef]
- Sahni, V.; Blackledge, T.A.; Dhinojwala, A. A Review on Spider Silk Adhesion. J. Adhes. 2011, 87, 595–614. [Google Scholar] [CrossRef]
- Regassa, Y.; Lemu, H.G.; Sirrabizuh, B.; Rahimeto, S. Studies on the Geometrical Design of Spider Webs for Reinforced Composite Structures. J. Compos. Sci. 2021, 5, 57. [Google Scholar] [CrossRef]
- Su, I.; Buehler, M.J. Spider silk: Dynamic mechanics. Nat. Mater. 2016, 15, 1054–1055. [Google Scholar] [CrossRef]
- Malay, A.D.; Craig, H.C.; Chen, J.; Oktaviani, N.A.; Numata, K. Complexity of Spider Dragline Silk. Biomacromolecules 2022, 23, 1827–1840. [Google Scholar] [CrossRef]
- He, W.; Qian, D.; Wang, Y.; Zhang, G.; Cheng, Y.; Hu, X.; Wen, K.; Wang, M.; Liu, Z.; Zhou, X.; et al. A Protein-Like Nanogel for Spinning Hierarchically Structured Artificial Spider Silk. Adv. Mater. 2022, 34, 2201843. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A review on advances in the applications of spider silk in biomedical issues. Int. J. Biol. Macromol. 2021, 192, 258–271. [Google Scholar] [CrossRef]
- Bittencourt, D.M.D.C.; Oliveira, P.; Michalczechen-Lacerda, V.A.; Rosinha, G.M.S.; Jones, J.A.; Rech, E.L. Bioengineering of spider silks for the production of biomedical materials. Front. Bioeng. Biotechnol. 2022, 10, 958486. [Google Scholar] [CrossRef]
- Humenik, M.; Pawar, K.; Scheibel, T. Nanostructured, Self-Assembled Spider Silk Materials for Biomedical Applications. In Biological and Bio-inspired Nanomaterials; Springer: Singapore, 2019; Volume 1174, pp. 187–221. [Google Scholar] [CrossRef]
- Kluge, J.A.; Rabotyagova, O.; Leisk, G.G.; Kaplan, D.L. Spider silks and their applications. Trends Biotechnol. 2008, 26, 244–251. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Huang, J.; Khan, A.Q.; An, B.; Zhou, X.; Liu, Z.; Zhu, M. Spider Silk-Inspired Artificial Fibers. Adv. Sci. 2022, 9, 202103965. [Google Scholar] [CrossRef]
- Zheng, K.; Ling, S. De Novo Design of Recombinant Spider Silk Proteins for Material Applications. Biotechnol. J. 2019, 14, e1700753. [Google Scholar] [CrossRef] [Green Version]
- Ramezaniaghdam, M.; Nahdi, N.D.; Reski, R. Recombinant Spider Silk: Promises and Bottlenecks. Front. Bioeng. Biotechnol. 2022, 10, 835637. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, G.; Oliveira, P.; Krishnaji, S.T.; Chen, D.; Hinman, M.; Bell, B.; Harris, T.I.; Ghazitabatabaei, A.; Lewis, R.V.; Jones, J.A. Large scale production of synthetic spider silk proteins in Escherichia coli. Protein Expr. Purif. 2021, 183, 105839. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debabov, V.G.; Bogush, V.G. Recombinant Spidroins as the Basis for New Materials. ACS Biomater. Sci. Eng. 2020, 6, 3745–3761. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Jimenez, V.M.; Dudley, R. Spiderweb deformation induced by electrostatically charged insects. Sci. Rep. 2013, 3, 2108. [Google Scholar] [CrossRef] [Green Version]
- Vollrath, F.; Edmonds, D. Consequences of electrical conductivity in an orb spider’s capture web. Naturwissenschaften 2013, 100, 1163–1169. [Google Scholar] [CrossRef]
- Mulder, T.; Mortimer, B.; Vollrath, F. Functional flexibility in a spider’s orb web. J. Exp. Biol. 2020, 223 Pt 23, jeb234070. [Google Scholar] [CrossRef]
- Opell, B.D.; Hendricks, M.L. Adhesive recruitment by the viscous capture threads of araneoid orb-weaving spiders. J. Exp. Biol. 2007, 210 Pt 4, 553–560. [Google Scholar] [CrossRef]
- Opell, B.D.; Schwend, H.S. Adhesive efficiency of spider prey capture threads. Zoology 2009, 112, 16–26. [Google Scholar] [CrossRef]
- Opell, B.D.; Jain, D.; Dhinojwala, A.; Blackledge, T.A. Tuning orb spider glycoprotein glue performance to habitat humidity. J. Exp. Biol. 2018, 221 Pt 6, jeb161539. [Google Scholar] [CrossRef] [Green Version]
- Tarakanova, A.; Buehler, M.J. The role of capture spiral silk properties in the diversification of orb webs. J. R. Soc. Interface 2012, 9, 3240–3248. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.-H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef]
- Kinahan, M.E.; Filippidi, E.; Köster, S.; Hu, X.; Evans, H.M.; Pfohl, T.; Kaplan, D.L.; Wong, J. Tunable Silk: Using Microfluidics to Fabricate Silk Fibers with Controllable Properties. Biomacromolecules 2011, 12, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, J.; Rosei, F. Photonic crystals: Sustainable sensors from silk. Nat. Mater. 2013, 12, 98–100. [Google Scholar] [CrossRef]
- Schneider, D.; Gomopoulos, N.; Koh, C.Y.; Papadopoulos, P.; Kremer, F.; Thomas, E.L.; Fytas, G. Nonlinear control of high-frequency phonons in spider silk. Nat. Mater. 2016, 15, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shao, Z.; Vollrath, F. Relationships between supercontraction and mechanical properties of spider silk. Nat. Mater. 2005, 4, 901–905. [Google Scholar] [CrossRef]
- Becker, N.; Oroudjev, E.; Mutz, S.; Cleveland, J.P.; Hansma, P.K.; Hayashi, C.Y.; Makarov, D.E.; Hansma, H.G. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003, 2, 278–283. [Google Scholar] [CrossRef]
- Belbéoch, C.; Lejeune, J.; Vroman, P.; Salaün, F. Silkworm and spider silk electrospinning: A review. Environ. Chem. Lett. 2021, 19, 1737–1763. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Wen, X.-H.; Sun, P.; Zeng, C.; Liu, M.-Y.; Yang, F.; Bi, H.; Li, D.; Ma, R.-G.; Wang, J.-C.; et al. Spider Web-like Flexible Tactile Sensor for Pressure-Strain Simultaneous Detection. ACS Appl. Mater. Interfaces 2021, 13, 10428–10436. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-Web-Inspired PM0.3 Filters Based on Self-Sustained Electrostatic Nanostructured Networks. Adv. Mater. 2020, 32, e2002361. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Tang, N.; Ali, N.; Yu, J.; Ding, B. Highly Efficient, Transparent, and Multifunctional Air Filters Using Self-Assembled 2D Nanoarchitectured Fibrous Networks. ACS Nano 2019, 13, 13501–13512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Tang, N.; Ge, J.; Yu, J.; Ding, B. Direct electronetting of high-performance membranes based on self-assembled 2D nanoarchitectured networks. Nat. Commun. 2019, 10, 1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Guo, X.; Chu, R.; Wang, S.; Zeng, W.; Qu, L.; Zhao, Y.; Yan, F.; Xing, G. Rapid-Response, Low Detection Limit, and High-Sensitivity Capacitive Flexible Tactile Sensor Based on Three-Dimensional Porous Dielectric Layer for Wearable Electronic Skin. ACS Appl. Mater. Interfaces 2019, 11, 40716–40725. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, L.; Zurovec, M.; Kludkiewicz, B.; Hradilova, M.; Strnad, H.; Sehnal, F. Modular structure, sequence diversification and appropriate nomenclature of seroins produced in the silk glands of Lepidoptera. Sci. Rep. 2019, 9, 3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakimi, O.; Knight, D.P.; Vollrath, F.; Vadgama, P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007, 38, 324–337. [Google Scholar] [CrossRef]
- Pereira, R.F.P.; Silva, M.M.; de Zea Bermudez, V. Bombyx moriSilk Fibers: An Outstanding Family of Materials. Macromol. Mater. Eng. 2014, 300, 1171–1198. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-chain, L-chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Sun, S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Shen, X.; Shi, H.; Wei, H.; Wu, B.; Xia, Q.; Yeo, J.; Huang, W. Engineering Natural and Recombinant Silks for Sustainable Bio-devices. Front. Chem. 2022, 10, 881028. [Google Scholar] [CrossRef]
- Dyakonov, T.; Yang, C.H.; Bush, D.; Gosangari, S.; Majuru, S.; Fatmi, A. Design and Characterization of a Silk-Fibroin-Based Drug Delivery Platform Using Naproxen as a Model Drug. J. Drug Deliv. 2012, 2012, 490514. [Google Scholar] [CrossRef] [Green Version]
- Ghalei, S.; Handa, H. A review on antibacterial silk fibroin-based biomaterials: Current state and prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef]
- Lehmann, T.; Vaughn, A.E.; Seal, S.; Liechty, K.W.; Zgheib, C. Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 2022, 14, 651. [Google Scholar] [CrossRef]
- Sultan, T.; Hong, H.; Lee, O.J.; Ajiteru, O.; Lee, Y.J.; Lee, J.S.; Lee, H.; Kim, S.H.; Park, C.H. Silk Fibroin-Based Biomaterials for Hemostatic Applications. Biomolecules 2022, 12, 660. [Google Scholar] [CrossRef]
- Yang, C.; Li, S.; Huang, X.; Chen, X.; Shan, H.; Chen, X.; Tao, L.; Zhang, M. Silk Fibroin Hydrogels Could Be Therapeutic Bio-materials for Neurological Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 2076680. [Google Scholar]
- Asakura, T. Structure of Silk I (Bombyx mori Silk Fibroin before Spinning) -Type II β-Turn, Not α-Helix. Molecules 2021, 26, 3706. [Google Scholar] [CrossRef]
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of silk III at the air-water interface. Int. J. Biol. Macromol. 1999, 24, 237–242. [Google Scholar] [CrossRef]
- Cao, T.-T.; Zhang, Y.-Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Dong, Z.; Xia, Q.; Zhao, P. Antimicrobial components in the cocoon silk of silkworm, Bombyx mori. Int. J. Biol. Macromol. 2022, in press. [Google Scholar] [CrossRef]
- Nirmala, X.; Mita, K.; Vanisree, V.; Zurovec, M.; Sehnal, F. Identification of four small molecular mass proteins in the silk of Bombyx mori. Insect Mol. Biol. 2001, 10, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Vaishna, R.L.; Kakkar, A.; Arunkumar, K.P.; Nagaraju, J. Characterization of antiviral and antibacterial activity of Bombyx mori seroin proteins. Cell. Microbiol. 2014, 16, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, X.; Lu, M.; Chen, H.; Chen, S.; Han, J.; Zhang, Y.; Zhao, P.; Dong, Z. Antibacterial Mechanism of Silkworm Seroins. Polymers 2020, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Waite, J. Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis L. J. Biol. Chem. 1983, 258, 2911–2915. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Waite, J.H. Mussel adhesion—Essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Petrone, L.; Tan, Y.; Cai, H.; Israelachvili, J.N.; Miserez, A.; Waite, J.H. An Underwater Surface-Drying Peptide Inspired by a Mussel Adhesive Protein. Adv. Funct. Mater. 2016, 26, 3496–3507. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Bian, X.; Bu, Y. Applications of Bioadhesives: A Mini Review. Front. Bioeng. Biotechnol. 2021, 9, 716035. [Google Scholar] [CrossRef]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Weiser, T.; Regenbogen, S.E.; Thompson, K.D.; Haynes, B.; Lipsitz, S.R.; Berry, W.R.; Gawande, A.A. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet 2008, 372, 139–144. [Google Scholar] [CrossRef]
- Weiser, T.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Estimate of the global volume of surgery in 2012: An assessment supporting improved health outcomes. Lancet 2015, 385 (Suppl. S2), S11. [Google Scholar] [CrossRef]
- Dobson, G.P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef]
- Dhandapani, V.; Ringuette, V.; Desrochers, M.; Sirois, M.; Vermette, P. Composition, host responses and clinical applications of bioadhesives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 12, 2779–2797. [Google Scholar] [CrossRef]
- Lee, Y.; Xu, C.; Sebastin, M.; Lee, A.; Holwell, N.; Xu, C.; Miranda Nieves, D.; Mu, L.; Langer, R.S.; Lin, C.; et al. Bioinspired Nanoparticulate Medical Glues for Minimally Invasive Tissue Repair. Adv. Healthc. Mater. 2015, 4, 2587–2596. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.D.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E.; et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired multilayer membranes as po-tential adhesive patches for skin wound healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.-E.; Im, B.G.; Kim, J.-S.; Jang, J.-H. Multifunctional Self-Adhesive Fibrous Layered Matrix (FiLM) for Tissue Glues and Therapeutic Carriers. Biomacromolecules 2017, 18, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Hwang, J.J.; Stupp, S.I. Poly(amino acid) bioadhesives for tissue repair. J. Biomater. Sci. Polym. Ed. 2000, 11, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.V. Current state and use of biological adhesives in orthopedic surgery. Orthopedics 2014, 37, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, D.S.; Gim, Y.; Cha, H.J. Expression of Functional Recombinant Mussel Adhesive Protein Type 3A in Escherichia coli. Biotechnol. Prog. 2005, 21, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Pardeshi, S.; Sharma, J.G.; Lee, S.H.; Choi, E.H. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar. Drugs 2015, 13, 6792–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulman, S.E.; Coleman, C.M.; Murphy, J.M.; Medcalf, N.; Ryan, A.E.; Barry, F. Pullulan: A new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res. Ther. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Suchaoin, W.; Bonengel, S.; Griessinger, J.A.; Pereira de Sousa, I.; Hussain, S.; Huck, C.W.; Bernkop-Schnürch, A. Novel bioadhesive polymers as intra-articular agents: Chondroitin sulfate-cysteine conjugates. Eur. J. Pharm. Biopharm. 2016, 101, 25–32. [Google Scholar] [CrossRef]
- Laulicht, B.; Mancini, A.; Geman, N.; Cho, D.; Estrellas, K.; Furtado, S.; Hopson, R.; Tripathi, A.; Mathiowitz, E. Bioinspired Bioadhesive Polymers: Dopa-Modified Poly(acrylic acid) Derivatives. Macromol. Biosci. 2012, 12, 1555–1565. [Google Scholar] [CrossRef]
- Hwang, D.S.; Gim, Y.; Kang, D.G.; Kim, Y.K.; Cha, H.J. Recombinant mussel adhesive protein Mgfp-5 as cell adhesion biomaterial. J. Biotechnol. 2007, 127, 727–735. [Google Scholar] [CrossRef]
- Hwang, D.S.; Yoo, H.J.; Jun, J.H.; Moon, W.K.; Cha, H.J. Expression of Functional Recombinant Mussel Adhesive Protein Mgfp-5 in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 3352–3359. [Google Scholar] [CrossRef]
- Kim, E.; Dai, B.; Qiao, J.B.; Li, W.; Fortner, J.D.; Zhang, F. Microbially Synthesized Repeats of Mussel Foot Protein Display Enhanced Underwater Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 43003–43012. [Google Scholar] [CrossRef]
- Greer, N.; Foman, N.; Dorrian, J.; Fitzgerald, P.; Macdonald, R.; Rutks, I.; Wilt, T. Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review. Ann. Intern. Med. 2013, 159, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Baudner, B.C.; O’Hagan, D.T. Bioadhesive delivery systems for mucosal vaccine delivery. J. Drug Target. 2010, 18, 752–770. [Google Scholar] [CrossRef]
- Coucke, D.; Schotsaert, M.; Libert, C.; Pringels, E.; Vervaet, C.; Foreman, P.; Saelens, X.; Remon, J. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine 2009, 27, 1279–1286. [Google Scholar] [CrossRef]
- Singh, M.; Briones, M.; O’Hagan, D.T. A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J. Control Release 2001, 70, 267–276. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Lin, C.B.; Lee, Y.-T.; Liu, C.-Y. Optimal photonic nanojet beam shaping by mesoscale dielectric dome lens. J. Appl. Phys. 2020, 127, 243110. [Google Scholar] [CrossRef]
- Khuyen, N.; Han, P.V.D.; Nguyen, N.T.; Le, Q.B.; Harjo, M.; Anbarjafari, G.; Kiefer, R.; Tamm, T. The Use of Laminates of Com-mercially Available Fabrics for Anti-Stab Body-Armor. Polymers 2021, 13, 1077. [Google Scholar] [CrossRef]
- Hamidi, Y.; Yalcinkaya, M.A.; Guloglu, G.E.; Pishvar, M.; Amirkhosravi, M.; Altan, M.C. Silk as a Natural Reinforcement: Pro-cessing and Properties of Silk/Epoxy Composite Laminates. Materials 2018, 11, 2135. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Zhu, W.; Peck, Y.; Iqbal, J.; Wang, D.-A. A novel DOPA-albumin based tissue adhesive for internal medical applications. Biomaterials 2017, 147, 99–115. [Google Scholar] [CrossRef]
- Zhu, W.; Chuah, Y.J.; Wang, D.-A. Bioadhesives for internal medical applications: A review. Acta Biomater. 2018, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]







| Common Name | Species | Protein | NCBI Entry | Ref. |
|---|---|---|---|---|
| Barnacle | Megabalanus rosa | Mrcp-19k | BAE94409 | [91] |
| Mrcp-20k | BAB18762 | [92] | ||
| Mrcp-52k | BAL22342 | [93] | ||
| Mrcp-100k | BAB12269 | [94] | ||
| Balanus albicostatus | Balcp-19k | AB242295 | [91] | |
| Balcp-20k | AB329666 | [95] | ||
| Balanus improvisus | Bicp-19k | AB242296 | [91] | |
| Spider | Nephila clavipes | ASG1 | EU780014 | [96] |
| ASG2 | EU780015 | [96] | ||
| PySp2 | HM020705 | [97] | ||
| Latrodectus hesperus | AgSF1 | JX262195 | [98] | |
| PySp1 | FJ973621 | [99] | ||
| Mussel | Dreissena polymorpha | Dpfp1 | AAF75279 | [100] |
| Dpfp2 | AM229730 | [101] | ||
| Mytilus californianus | Mfp-3S | DQ165556 | [102] | |
| Mcfp-5 | DQ444853 | [103] | ||
| Mcfp-6 | DQ351537 | [104] | ||
| Mytilus edulis | Mefp-1 | AY845258 | [105] | |
| Mefp-3 | AF286136 | [105] | ||
| Mefp-5 | AAL35297 | [106] | ||
| Mytilus galloprovincialis | Mgfp1 | D63778 | [107] | |
| Mgfp5 | AY521220 | [108] | ||
| Perna viridis | Pvfp-1 | AAY46226 | [109] | |
| Pvfp-2 | AGZ84282 | [97] | ||
| Pvfp-3 | AGZ84285 | [97] | ||
| Pvfp-5 | AGZ84279 | [97] | ||
| Pvfp-6 | AGZ84283 | [97] | ||
| Slug | Lehmannia valentiana | Sm40 | ABR68007 | [110] |
| Sm85 | ABR68008 | [110] | ||
| Tubeworm | Phragmatopoma californica | Pc-1 | AAY29115 | [111] |
| Pc-2 | AAY29116 | [111] | ||
| Pc-3A | AY960618 | [111] | ||
| Pc-3B | AY960621 | [112] | ||
| Pc-4 | GH160602 | [112] | ||
| Pc-5 | GH160603 | [112] | ||
| Sabellaria alveolata | Sa-1 | CCD57439 | [113] | |
| Sa-2 | CCD57460 | [113] | ||
| Sa-3A | CCD57480 | [113] | ||
| Sa-3B | CCD57502 | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melrose, J. High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules 2022, 27, 8982. https://doi.org/10.3390/molecules27248982
Melrose J. High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules. 2022; 27(24):8982. https://doi.org/10.3390/molecules27248982
Chicago/Turabian StyleMelrose, James. 2022. "High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired" Molecules 27, no. 24: 8982. https://doi.org/10.3390/molecules27248982
APA StyleMelrose, J. (2022). High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules, 27(24), 8982. https://doi.org/10.3390/molecules27248982