Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assimilation of Sugars in the Molasses by C. ljungdahlii DSM 13528 for 2,3-BDO Production
2.2. Replacement of Ingredients in the Modified DSMZ 879 Medium Using Molasses
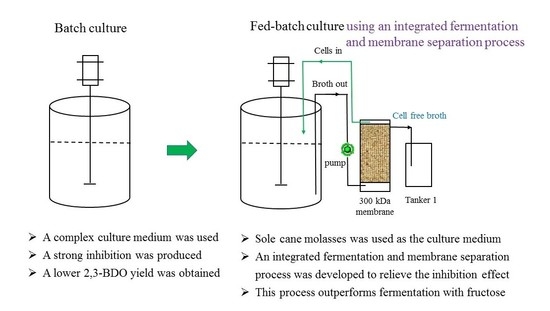
2.3. Effect of Batch and Fed-Batch Cultures on 2,3-BDO Production with the Sole Molasses as the Substrate
2.4. Effect of the Inhibitory Factors on 2,3-BDO Production with Molasses Alone as the Substrate
2.5. Production of 2,3-BDO Using an Integrated Fermentation and Membrane Separation Process
3. Materials and Methods
3.1. Microorganism, Media, and Cultivation Conditions
3.2. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Oh, A.; Em, B.; Bhl, C. The current strategies and parameters for the enhanced microbial production of 2,3-butanediol. Biotechnol. Rep. 2020, 25, e00397. [Google Scholar]
- Kopke, M.; Mihalcea, C.; Liew, F.; Tizard, J.; Ali, M.; Conolly, J.; Al-Sinawi, B.; Simpson, S. 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dgk, A.; Swy, A.; Mk, A.; Jkk, B.; Yubc, D.; Mko, A. Improved 2,3-butanediol yield and productivity from lignocellulose biomass hydrolysate in metabolically engineered Enterobacter Aerogenes. Bioresour. Technol. 2020, 309, 123386. [Google Scholar]
- Ji, X.J.; He, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Prabhu, A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Tinco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D. Technological development of the bio-based 2,3-butanediol process. Biofuels Bioprod. Biorefining 2021, 15, 357–376. [Google Scholar] [CrossRef]
- Rehman, S.; Khairul Islam, M.; Khalid Khanzada, N.; Kyoungjin An, A.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Rao, B.; Liao, Y.Z.; Sun, J.; Gang, S.; Wei, D.; Chu, J.; Zhu, J.; Shen, Y. Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens. Appl. Microbiol. Biotechnol. 2012, 93, 2147–2159. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Wang, A.; Yu, W.; Jiang, T.; Li, L.; Ma, C.; Xu, P. Production of 2,3-butanediol from corncob molasses, a waste by-product in xylitol production. Appl. Microbiol. Biotechnol. 2010, 87, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Liu, Q.; Zhang, J.A.; Li, J.P.; Xu, J.M.; Wang, G.H. Improved 2,3-butanediol production from corncob acid hydrolysate by fed-batch fermentation using Klebsiella oxytoca. Process. Biochem. 2010, 45, 613–616. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Fang, Z.; Guo, F.; Yang, L.B. Production of 2,3-butanediol from acid hydrolysates of Jatropha hulls with Klebsiella oxytoca. Bioresour. Technol. 2012, 107, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Wang, X.D.; Dai, J.Y.; Xiu, Z.L. Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Y.; Zhao, P.; Cheng, X.-L.; Xiu, Z.-L. Enhanced production of 2,3-butanediol from sugarcane molasses. Appl. Biochem. Biotechnol. 2015, 175, 3014–3024. [Google Scholar] [CrossRef]
- Jung, M.Y.; Jung, H.M.; Lee, J.; Oh, M.K. Alleviation of carbon catabolite repression in Enterobacter aerogenes for efficient utilization of sugarcane molasses for 2,3-butanediol production. Biotechnol. Biofuels 2015, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afschar, A.S.; Bellgardt, K.H.; Rossell, C.; Czok, A.; Schaller, K. The production of 2,3-butanediol by fermentation of high test molasses. Appl. Microbiol. Biotechnol. 1991, 34, 582–585. [Google Scholar] [CrossRef]
- Li, L.; Li, K.; Wang, Y.; Chen, C.; Xu, Y.; Zhang, L.; Han, B.; Gao, C.; Tao, F.; Ma, C.; et al. Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab. Eng. 2015, 28, 19–27. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Succinic acid biosynthesis from cane molasses under low pH by Actinobacillus succinogenes immobilized in luffa sponge matrices. Bioresour. Technol. 2018, 268, 45–51. [Google Scholar] [CrossRef]
- Luo, J.; Guo, S.; Wu, Y.; Wan, Y. Separation of sucrose and reducing sugar in cane molasses by nanofiltration. Food Bioproc. Technol. 2018, 11, 913–925. [Google Scholar] [CrossRef]
- Tinoco, D.; Castro, A.; Seldin, L.; Freire, D. Production of (2R,3R)-butanediol by Paenibacillus polymyxa PM 3605 from crude glycerol supplemented with sugarcane molasses. Process. Biochem. 2021, 106, 88–95. [Google Scholar] [CrossRef]
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S. Fermentation of biodiesel-derived glycerol by Bacillus amyloliquefaciens: Effects of co-substrates on 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2013, 97, 7651–7658. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, V.; Vicente, G.D.; Moran, B.; Rojas, A.; Segarra, S.; Montesinos, A.; Tortajada, M.; Ramon, D.; Ladero, M.; Santos, V.E. Novel biocatalysts for glycerol conversion into 2,3-butanediol. Process Biochem. 2016, 51, 740–748. [Google Scholar] [CrossRef]
- Zhu, H.F.; Liu, Z.Y.; Zhou, X.; Yi, J.H.; Lun, Z.M.; Wang, S.N.; Tang, W.Z. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii. Front. Microbiol. 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Whitham, J.; Pawlak, J.; Grunden, A. Clostridium ljungdahlii: A review of the development of an industrial biocatalyst. Curr. Biotechnol. 2015, 5, 54–70. [Google Scholar]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Bacteriol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Luo, J.; Wu, Y.; Qi, B.; Chen, X.; Wan, Y. Decoloration of sugarcane molasses by tight ultrafiltration: Filtration behavior and fouling control. Sep. Purif. Technol. 2018, 204, 66–74. [Google Scholar] [CrossRef]
- Guo, S.; Luo, J.; Yang, Q.; Qiang, X.; Feng, S.; Wan, Y. Decoloration of molasses by ultrafiltration and nanofiltration: Unraveling the mechanisms of high sucrose retention. Food Bioproc. Technol. 2018, 12, 39–53. [Google Scholar] [CrossRef]
- Cao, W.; Luo, J.; Qi, B.; Zhao, J.; Qiao, C.; Ding, L.; Su, Y.; Wan, Y. β-poly(l-malic acid) production by fed-batch culture of Aureobasidium pullulans ipe-1 with mixed sugars. Eng. Life Sci. 2014, 14, 180–189. [Google Scholar] [CrossRef]
- Xiao, Z.; Ping, X. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef]
- Nakashimada, Y.; Marwoto, B.; Kashiwamura, T.; Kakizono, T.; Nishio, N. Enhanced 2,3-butanediol production by addition of acetic acid in Paenibacillus polymyxa. J. Biosci. Bioeng. 2000, 90, 661–664. [Google Scholar] [CrossRef]
- Mayer, D.; Schlensog, V.; Bock, A. Identification of the transcriptional activator. J. Bacteriol. 1995, 177, 5261–5269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryn, K.; Ulstrup, J.C.; Størmer, F.C. Effect of acetate upon the formation of acetoin in Klebsiella and Enterobacter and itspPossible practical application in a rapid voges-proskauer test. Appl. Environ. Microbiol. 1973, 25, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour. Technol. 2018, 266, 26–33. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.; Cai, D.; Wang, B.; Qin, P.; Wang, Z.; Tan, T. Improvement of l-lactic acid productivity from sweet sorghum juice by repeated batch fermentation coupled with membrane separation. Bioresour. Technol. 2016, 211, 291–297. [Google Scholar] [CrossRef]
- Cao, W.F.; Luo, J.Q.; Zhao, J.; Qiao, C.S.; Ding, L.H.; Qi, B.K.; Su, Y.; Wan, Y.H. Intensification of β-poly(L-malic acid) production by Aureobasidium pullulans ipe-1 in the late exponential growth phase. J. Ind. Microbiol. Biotechnol. 2012, 39, 1073–1080. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Qi, B.; Luo, J.; Zhang, Y.; Su, Y.; Wan, Y. Efficient production of acetone–butanol–ethanol (ABE) from cassava by a fermentation–pervaporation coupled process. Bioresour. Technol. 2014, 169, 251–257. [Google Scholar] [CrossRef]
- Siemerink, M.; Kuit, W.; Contreras, A.; Eggink, A.; Eggink, G.; Oost, J.; Kengen, A. D-2,3-Butanediolproduction due to heterologous expression of an acetoin reductase in Clostridium acetobutylicum. Appl. Environ. Microb. 2011, 77, 2582–2588. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Liu, J.; Liu, Z.; Li, F. Characterization of two novel butanol dehydrogenases involved in butanol degradation in syngas-utilizing bacterium Clostridium ljungdahlii DSM 13528. J. Basic Microbiol. 2014, 54, 996–1004. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, Y.; Park, Y.; Seo, J. Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Cs, A.; Rc, A.; Vsabc, D.; Jsa, E. Engineering of the 2,3-butanediol pathway of Paenibacillus polymyxa DSM 365. Metab. Eng. 2020, 61, 381–388. [Google Scholar]
- Cui, J.; Li, H.; Luo, J.; Cao, W.; Qiao, C.; Wan, Y. One step separation of Aureobasidium pullulans from beta-poly(L-malic acid) fermentation broth by membranes technology. J. Chem. Technol. Biotechnol. 2017, 92, 845–853. [Google Scholar] [CrossRef]
- Lim, A.L.; Bai, R. Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J. Membr. Sci. 2003, 216, 279–290. [Google Scholar] [CrossRef]
- Priya, A.; Dureja, P.; Rathi, R.; Lal, B. Comparative assessment of separation techniques for downstream processing of 2,3-Butanediol. Fuel 2021, 292, 120351. [Google Scholar] [CrossRef]









| Medium a | Components |
| M1 b | 35 g/L total sugars from molasses; 1.0 g/L NH4Cl, 0.1 g/L KCl, 0.2 g/L MgSO4·7 H2O, 0.8 g/L NaCl, 0.02 g/L CaCl2·2 H2O, 0.1 g KH2PO4, 2.5 mg/L Na2WO4·2 H2O, 1.0 g/L NaHCO3, 1.0 g/L cysteine-HCl·H2O, 1 g/L yeast extract, 0.5 g/L cysteine, 0.5 mg/L resazurin (i.e., Part A in the modified DSMZ 879 medium). |
| M2 | 35 g/L total sugars from molasses; 10 mL vitamin solution (i.e., Part B in the modified DSMZ 879 medium). The vitamin solution contains 2 mg biotin, 2 mg folic acid, 10 mg pyridoxine-HCl, 25 mg thiamine-HCl·2 H2O, 5 mg riboflavin, 5 mg nicotinic acid, 5 mg d-Ca-pantothenate, 0.1 mg vitamin B12, 5 mg ρ-aminobenzoic acid, and 5 mg lipoic acid in 1 L distilled water |
| M3 | 35 g/L total sugars from molasses; 10 mL trace element solution (i.e., Part C in the modified DSMZ 879 medium). The trace element solution contains 2.0 g nitrilotriacetic acid, 1.3 g MnCl2·H2O, 0.4 g FeSO4·7 H2O, 0.2 g CoCl2·7 H2O, 0.2 g ZnSO4·7 H2O, 0.2 g Na2MoO4·2 H2O, 0.02 g NiCl2·6 H2O, and 0.1 g Na2SeO3·5 H2O in 1 L distilled water. |
| M4 | 35 g/L total sugars from molasses; Part A, Part B, Part C in the modified DSMZ 879 medium |
| M5 | 35 g/L total sugars from molasses; 1.0 g/L NH4Cl, 0.1 g/L KCl, 0.2 g/L MgSO4·7 H2O, 0.8 g/L NaCl, 0.02 g/L CaCl2·2 H2O, 0.1 g KH2PO4, 2.5 mg/L Na2WO4·2 H2O, 1.0 g/L NaHCO3, 1 g/L yeast extract, 0.5 mg/L resazurin (Part A), 10 mL trace element solution (Part B), and 10 mL vitamin solution (Part C) in the modified DSMZ 879 medium |
| M6 | The molasses contained a final concentration of 35 g/L total sugars |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Deng, T.; Cao, W.; Shen, F.; Liu, S.; Zhang, J.; Liang, X.; Wan, Y. Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process. Molecules 2022, 27, 954. https://doi.org/10.3390/molecules27030954
Yang Y, Deng T, Cao W, Shen F, Liu S, Zhang J, Liang X, Wan Y. Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process. Molecules. 2022; 27(3):954. https://doi.org/10.3390/molecules27030954
Chicago/Turabian StyleYang, Yuling, Tingting Deng, Weifeng Cao, Fei Shen, Sijia Liu, Jing Zhang, Xinquan Liang, and Yinhua Wan. 2022. "Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process" Molecules 27, no. 3: 954. https://doi.org/10.3390/molecules27030954
APA StyleYang, Y., Deng, T., Cao, W., Shen, F., Liu, S., Zhang, J., Liang, X., & Wan, Y. (2022). Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process. Molecules, 27(3), 954. https://doi.org/10.3390/molecules27030954