The Theoretical and Experimental Investigation of the Fluorinated Palladium β-Diketonate Derivatives: Structure and Physicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
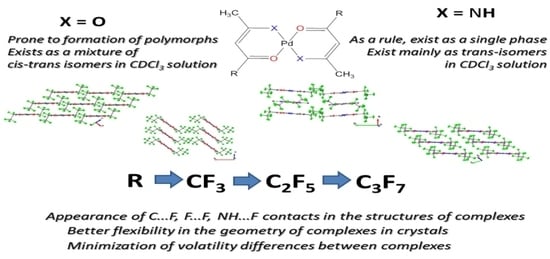
2.1. Characterization of Complexes 1–6
2.2. Single-Crystal and Powder XRD Analyses
2.3. Hirshfeld Surface Analysis of Complexes 1–6
2.4. Lattice Energy Calculations of the Complexes 1–6
2.5. Thermal Properties of the Complexes 1–6
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nayebossadri, S.; Speight, J.D.; Book, D. Hydrogen separation from blended natural gas and hydrogen by Pd-based membranes. Int. J. Hydrogen Energy 2019, 44, 29092–29099. [Google Scholar] [CrossRef]
- Tosti, S.; Bettinali, L.; Violante, V. Rolled thin Pd and Pd–Ag membranes for hydrogen separation and production. Int. J. Hydrogen Energy 2000, 25, 319–325. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Zhang, X.; Yu, Q.-Y.; Liu, Z.-C.; Xu, C.-M.; Gao, J.-S.; Zhuang, J.; Wang, X. Pd Cluster Nanowires as Highly Efficient Catalysts for Selective Hydrogenation Reactions. Chem.–A Eur. J. 2012, 18, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs doped chitosan-based composite hydrogels as highly efficient catalysts for reduction of 4-nitrophenol. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 611, 125889. [Google Scholar] [CrossRef]
- Wu, Y.; Huo, X.; Zhang, W. Synergistic Pd/Cu Catalysis in Organic Synthesis. Chem.–A Eur. J. 2020, 26, 4895–4916. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Basova, T.V.; Ray, A.K. Review—Hybrid Materials Based on Phthalocyanines and Metal Nanoparticles for Chemiresistive and Electrochemical Sensors: A Mini-Review. ECS J. Solid State Sci. Technol. 2020, 9, 061001. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Schnabel, R.; Fijten, R.; Smolinska, A.; Dallinga, J.W.; Boumans, M.L.; Stobberingh, E.; Boots, A.W.; Roekaerts, P.M.H.J.; Bergmans, D.C.J.J.; Van Schooten, F.J. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci. Rep. 2015, 5, 17179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Yang, Z.; Navale, S.; Han, S.; Liu, X.; Liu, W.; Lu, Y.; Stadler, F.; Zhu, D. Ethanol sensing behavior of Pd-nanoparticles decorated ZnO-nanorod based chemiresistive gas sensors. Sens. Actuators B Chem. 2019, 298, 126850. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Improving the hydrogen sensing properties of SnO2 nanowire-based conductometric sensors by Pd-decoration. Sens. Actuators B Chem. 2019, 285, 358–367. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.; Suh, E.-K.; Hahn, Y.-B. Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloy Compd. 2019, 797, 456–464. [Google Scholar] [CrossRef]
- Kuchumov, B.; Koretskaya, T.; Igumenov, I.; Maksimovskii, E.; Voronov, Y. Monitoring the microstructure of nanosized palladium layers obtained via thermal and VUV stimulated MOCVD. Surf. Coat. Technol. 2013, 230, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Lyon, L.B.; Jiang, Y.-B.; Weimer, A.W. Scalable synthesis of palladium nanoparticle catalysts by atomic layer deposition. J. Nanoparticle Res. 2012, 14, 943. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Iatsunskyi, I.; Coy, E.; Renard, A.; Salameh, C.; Weber, M.; Sayegh, S.; Nada, A.; Roualdes, S.; et al. Atomic layer deposition of Pd nanoparticles on self-supported carbon-Ni/NiO-Pd nanofiber electrodes for electrochemical hydrogen and oxygen evolution reactions. J. Colloid Interface Sci. 2020, 569, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Noble Metals and Their Oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Cheng, Y.-C.; Tung, Y.-L.; Chi, Y.; Chen, Y.-L.; Liu, C.-S.; Peng, S.-M.; Lee, G.-H. Synthesis and characterization of fluorinated β-ketoiminate and imino-alcoholate Pd complexes: Precursors for palladium chemical vapor deposition. J. Mater. Chem. 2003, 13, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Assim, K.; Melzer, M.; Korb, M.; Rüffer, T.; Jakob, A.; Noll, J.; Georgi, C.; Schulz, S.E.; Lang, H. Bis(β-diketonato)- and allyl-(β-diketonato)-palladium(ii) complexes: Synthesis, characterization and MOCVD application. RSC Adv. 2016, 6, 102557–102569. [Google Scholar] [CrossRef] [Green Version]
- Zharkova, G.I.; Stabnikov, P.A.; Sysoev, S.A.; Igumenov, I.K. Volatility and crystal lattice energy of palladium(II) chelates. J. Struct. Chem. 2005, 46, 320–327. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Puukilainen, E.; Sajavaara, T.; Ritala, M.; Leskela, M. Low temperature atomic layer deposition of noble metals using ozone and molecular hydrogen as reactants. Thin Solid Films 2013, 531, 243–250. [Google Scholar] [CrossRef]
- Zharkova, G.; Stabnikov, P.; Baidina, I.; Smolentsev, A.; Tkachev, S. Synthesis, properties and crystal structures of volatile β-ketoiminate Pd complexes, precursors for palladium chemical vapor deposition. Polyhedron 2009, 28, 2307–2312. [Google Scholar] [CrossRef]
- Shushanyan, A.D.; Nikolaeva, N.S.; Vikulova, E.S.; Zelenina, L.N.; Trubin, S.V.; Sysoev, S.V.; Dorovskikh, S.I.; Morozova, N.B. Thermochemical study of new volatile palladium(II) and copper(II) β-ketohydrazonates for CVD application. J. Therm. Anal. Calorim. 2019, 136, 2341–2352. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Cherkasov, S.A.; Nikolaeva, N.S.; Smolentsev, A.I.; Sysoev, S.V.; Morozova, N.B. Thermal behavior of volatile palladium(II) complexes with tetradentate Schiff bases containing propylene-diimine bridge. J. Therm. Anal. Calorim. 2019, 135, 2573–2582. [Google Scholar] [CrossRef]
- Stabnikov, P.; Zharkova, G.; Baidina, I.; Tkachev, S.; Krisyuk, V.; Igumenov, I. Synthesis and properties of a new ketoiminate derivative of the 2,2,6,6-tetramethylheptane-3,5-dionate ligand to prepare volatile CVD precursors. Polyhedron 2007, 26, 4445–4450. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Ilyin, I.Y.; Kovaleva, E.A.; Piryazev, D.A.; Zelenina, L.N.; Sysoev, S.V.; Morozova, N.B.; Zherikova, K.V. “Vitruvian” precursor for gas phase deposition: Structural insights into iridium β-diketonate volatilities. Phys. Chem. Chem. Phys. 2021, 23, 9889–9899. [Google Scholar] [CrossRef] [PubMed]
- Zharkova, G.I.; Sysoev, S.V.; Baidina, I.A.; Stabnikov, P.A.; Igumenov, I.K. Vapor pressure and crystal structure of platinum(II) trans-bis-(trifluoroketoiminate). J. Struct. Chem. 2005, 46, 494–500. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Krisyuk, V.V.; Mirzaeva, I.V.; Komarov, V.Y.; Trubin, S.V.; Turgambaeva, A.E.; Morozova, N.B. The volatile trimethylplatinum(IV) complexes: Effect of β-diketonate substituents on thermal properties. Polyhedron 2020, 182, 114475. [Google Scholar] [CrossRef]
- Lin, W.; Nuzzo, R.G.; Girolami, G.S. Mechanistic Studies of Palladium Thin Film Growth from Palladium(II) β-Diketonates. 2. Kinetic Analysis of the Transmetalation Reaction of Bis(hexafluoroacetylacetonato)palladium(II) on Copper Surfaces. J. Am. Chem. Soc. 1996, 118, 5988–5996. [Google Scholar] [CrossRef]
- Hatanaka, M. Anionic Amidinourea^|^ndash;Metal(II) Tautomers: MN2O2 or MN4? Bull. Chem. Soc. Jpn. 2014, 88, 162–172. [Google Scholar] [CrossRef]
- Krasnov, P.O.; Mikhaleva, N.S.; Kuzubov, A.A.; Nikolaeva, N.S.; Zharkova, G.I.; Sheludyakova, L.A.; Morozova, N.B.; Basova, T.V. Prediction of the relative probability and the kinetic parameters of bonds breakage in the molecules of palladium MOCVD precursors. J. Mol. Struct. 2017, 1139, 269–274. [Google Scholar] [CrossRef]
- Kovaleva, E.A.; Kuzubov, A.A.; Vikulova, E.S.; Basova, T.V.; Morozova, N.B. Mechanism of dicarbonyl(2,4-pentanedionato)iridium(I) decomposition on iron surface and in gas phase: Complex experimental and theoretical study. J. Mol. Struct. 2017, 1146, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Zharkova, G.I.; Baidina, I.A.; Gromilov, S.A.; Igumenov, I.K. Synthesis and Properties of the Palladium (II) and Platinum (II) Complexes with Trifluoroiminoketone: Crystal and Molecular Structures of Bis (1, 1, 1-Trifluoro-4-Iminopentan-2-onato) palladium (II). Russ. J. Coord. Chem. 1998, 24, 109–113. [Google Scholar]
- Eremin, D.B.; Boiko, D.A.; Borkovskaya, E.V.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand. Catal. Sci. Technol. 2018, 8, 3073–3080. [Google Scholar] [CrossRef]
- Reilly, A.M.; Tkatchenko, A. Understanding the role of vibrations, exact exchange, and many-body van der Waals interactions in the cohesive properties of molecular crystals. J. Chem. Phys. 2013, 139, 024705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings 2021, 11, 78. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Sysoev, S.V.; Stabnikov, P.A.; Logvinenko, V.A.; Igumenov, I.K. Vapor pressure and crystal lattice energy of volatile palladium(II) β-iminoketonates. J. Therm. Anal. Calorim. 2011, 103, 381–385. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Stabnikov, P.A.; Grankin, V.M.; Semyannikov, P.P.; Igumenov, I.K. Palladium (II) β-diketonates: Volatility and energy of the crystal lattice. Russ. J. Coord. Chem. 2000, 26, 576–581. [Google Scholar]
- Zharkova, G.I.; Baidina, I.A.; Smolentsev, A.I.; Igumenov, I.K. Properties and structure of new volatile phenyl-containing β-diketonates of trimethylplatinum(IV): (CH3)3Pt(btfa)H2O, (CH3)3Pt(bac)Py, and initial complex [(CH3)3PtI]4. J. Struct. Chem. 2013, 54, 1083–1090. [Google Scholar] [CrossRef]
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N.; Oleynik, I.I. Composition determination of post-metallocene olefin polymerization catalysts. J. Struct. Chem. 2010, 51, 186–191. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; The University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Jayatilaka, D.; Grimwood, D.J. Tonto: A Fortran Based Object-Oriented System for Quantum Chemistry and Crystallography BT-Computational Science—ICCS 2003; Sloot, P.M.A., Abramson, D., Bogdanov, A.V., Gorbachev, Y.E., Dongarra, J.J., Zomaya, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 142–151. [Google Scholar]
- Philipsen, P.H.T.; te Velde, G.; Baerends, E.J.; Berger, J.A.; de Boeij, P.L.; Groeneveld, J.A.; Kadantsev, E.S.; Klooster, R.; Kootstra, F.; Romaniello, P.; et al. BAND 2014; SCM, Theoretical Chemistry, Vrije Universiteit: Amsterdam, The Netherlands, 2014; Available online: http//www.scm.com (accessed on 8 June 2020).
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF2017; SCM, Theoretical Chemistry, Vrije Universiteit: Amsterdam, The Netherlands, 2014; Available online: http//www.scm.com (accessed on 25 May 2020).
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]










| Graphical Illustration of [Pd(CH3CXCHCO(R))2] | Ligand | Abbreviations for Ligands | Abbreviations for Complexes | |
|---|---|---|---|---|
| R | X | |||
 | CF3 | O | tfa | 1 |
| NH | i-tfa | 2 | ||
| C2F5 | O | pfpa | 3 | |
| NH | i-pfpa | 4 | ||
| C3F7 | O | hfba | 5 | |
| NH | i-hfba | 6 | ||
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| ΔE, kJ/mol | 1.92 | 18.24 | 1.72 | 19.33 | 2.18 | 23.58 |
| cis:trans | 43:57 | 3:97 | 36:64 | - | 36:64 | - |
| Identification Code | 2 | 3_1 | 3-2 | 5_1 | 5_2 | 5_3 | 4 | 6 |
|---|---|---|---|---|---|---|---|---|
| Empirical formula | C10H10F6N2O2Pd | C12H8F10O4Pd | C12H8F10O4Pd | C14H8F14O4Pd | C14H8F14O4Pd | C14H8F14O4Pd | C12H10F10N2O2Pd | C14H10F14N2O2Pd |
| Formula weight | 410.60 | 512.58 | 512.58 | 612.60 | 612.60 | 612.60 | 510.62 | 610.64 |
| Temperature/K | 250.0 | 150 | 220.0 | 150.0 | 220 | 250.0 | 150 | 150.0 |
| Crystal system | monoclinic | triclinic | monoclinic | monoclinic | triclinic | triclinic | monoclinic | triclinic |
| Space group | P21/n | P-1 | P21/c | C2/c | P-1 | P-1 | P21/c | P-1 |
| a/Å | 11.3784(9) | 4.9017(2) | 10.8322(5) | 23.7565(8) | 5.7613(3) | 9.3089(2) | 6.7968(6) | 5.0366(6) |
| b/Å | 5.0152(4) | 6.9392(3) | 17.2176(8) | 9.0373(3) | 13.0127(5) | 10.5336(3) | 20.3999(16) | 9.4484(11) |
| c/Å | 11.6614(8) | 11.7607(5) | 9.2280(4) | 8.9907(3) | 14.4882(6) | 12.1779(3) | 11.6796(10) | 10.7492(11) |
| α/° | 90 | 73.869(2) | 90 | 90 | 64.951(2) | 101.0370(10) | 90 | 72.759(5) |
| β/° | 94.072(3) | 81.705(2) | 105.107(2) | 94.0550(10) | 79.866(2) | 109.5360(10) | 90.504(3) | 81.207(6) |
| γ/° | 90 | 85.553(2) | 90 | 90 | 78.423(2) | 107.0890(10) | 90 | 76.160(6) |
| Volume/Å3 | 663.78(9) | 379.96(3) | 1661.58(13) | 1925.42(11) | 958.92(8) | 1018.54(4) | 1619.4(2) | 472.51(9) |
| Z | 2 | 1 | 4 | 4 | 2 | 2 | 4 | 1 |
| ρcalcg/cm3 | 2.054 | 2.240 | 2.049 | 2.113 | 2.122 | 1.997 | 2.094 | 2.146 |
| μ/mm−1 | 1.475 | 1.355 | 1.239 | 1.117 | 1.121 | 1.056 | 1.266 | 1.133 |
| F(000) | 400.0 | 248.0 | 992.0 | 1184.0 | 592.0 | 592.0 | 992.0 | 296.0 |
| 2Θ range for data collection/° | 4.834 to 56.586 | 6.116 to 66.582 | 3.894 to 56.854 | 4.824 to 66.27 | 5.54 to 72.892 | 3.748 to 61.044 | 3.488 to 56.622 | 3.982 to 66.536 |
| Reflections collected | 6711 | 4812 | 18767 | 13865 | 18778 | 18514 | 15558 | 6959 |
| Independent reflections | 1647 [Rint = 0.0403, Rsigma = 0.0338] | 4812 [Rint = ?, Rsigma = 0.0363] | 4138 [Rint = 0.0395, Rsigma = 0.0351] | 3651 [Rint = 0.0282, Rsigma = 0.0256] | 9200 [Rint = 0.0251, Rsigma = 0.0289] | 6206 [Rint = 0.0262, Rsigma = 0.0288] | 4015 [Rint = 0.0763, Rsigma = 0.0875] | 3588 [Rint = 0.0506, Rsigma = 0.0794] |
| Data/restraints/parameters | 1647/0/102 | 4812/0/126 | 4138/0/246 | 3651/0/152 | 9200/0/385 | 6206/27/303 | 4015/8/258 | 3588/1/156 |
| Goodness-of-fit on F2 | 1.058 | 1.086 | 1.026 | 1.044 | 1.054 | 1.037 | 1.026 | 1.045 |
| Final R indexes (I ≥ 2σ (I)) | R1 = 0.0325, wR2 = 0.0832 | R1 = 0.0275, wR2 = 0.0671 | R1 = 0.0376, wR2 = 0.0898 | R1 = 0.0210, wR2 = 0.0537 | R1 = 0.0284, wR2 = 0.0724 | R1 = 0.0537, wR2 = 0.1631 | R1 = 0.0514, wR2 = 0.0856 | R1 = 0.0300, wR2 = 0.0630 |
| Final R indexes (all data) | R1 = 0.0461, wR2 = 0.0929 | R1 = 0.0283, wR2 = 0.0676 | R1 = 0.0546, wR2 = 0.0981 | R1 = 0.0254, wR2 = 0.0567 | R1 = 0.0372, wR2 = 0.0798 | R1 = 0.0736, wR2 = 0.1812 | R1 = 0.1266, wR2 = 0.1061 | R1 = 0.0357, wR2 = 0.0644 |
| Largest diff. peak/hole/e Å−3 | 1.02/−0.78 | 0.95/−0.55 | 0.89/−0.66 | 0.90/−0.69 | 0.80/−0.86 | 0.94/−0.58 | 1.24/−0.85 | 0.53/−1.20 |
| Parameter | Palladium β-Diketonates | Palladium β-Iminoketonates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3_1 | 3_2 | 5_1 | 5_2 | 5_3 | 2 [32] | 4 | 6 | |
| Pd–O/Å | 1.979/ 1.982 | 1.977/ 1.985 | 1.982–1.988 | 1.979–1.981 | 1.979–1.980 | 1.968–1.977 | 1.986 | 1.979–1.983 | 1.978 |
| Pd–N/Å | N/A | N/A | N/A | N/A | N/A | N/A | 1.983 | 1.973–1.980 | 1.979 |
| ∠O– Pd –O/° | 94.803 | 95.026 | 94.542/94.638 | 94.652 | 94.417/ 94.529 | 94.367/ 94.483 | N/A | N/A | N/A |
| ∠N– Pd –O/° | N/A | N/A | N/A | N/A | N/A | N/A | 92.645 | 93.131/93.421 | 92.946 |
| ∠ (two metallocycles)/° | 0 | 0 | 2.306 | 0 | 0 | 0 | 0 | 0 | 0 |
| ∠ stacking/° | 45.407 | N/A | 42.732 | 40.580 | 53.35 | 42.279/ 41.354 | 47.590 | 6.261/ 6.550 | 47.591 |
| ∠ within stack/° | 0 | N/A | 10.611 | 10.149 | 0 | 11.711 | 0 | 0.29 | 0 |
| distance between molecules in stack/Å | 3.362 | 3.589 | 3.357–3.440 | 3.414 | 3.439 | 3.444–3.494 | 3.382 | 3.394 | 3.397 |
| Pd…Pd/Å | 4.788 | 4.902 | 4.636 | 4.495 | 5.761 | 4.654 | 5.015 | 3.398 | 5.037 |
| Complex | EvdW, kJ/mol | Edef, kJ/mol | Elatt, kJ/mol |
|---|---|---|---|
| 1 | 139.5 | 10.8 | 128.7 |
| 2 | 134.4 | 36.2 | 98.2 |
| 3_1 | 132.0 | 5.3 | 126.7 |
| 3_2 | 130.4 | 56.9 | 73.5 |
| 4 | 149.5 | 28.4 | 121.2 |
| 5_1 | 167.2 | 5.9 | 161.4 |
| 5_2 | 138.2 | 5.5 | 132.6 |
| 5_3 | 134.8 | 17.1 | 117.7 |
| 6 | 150.6 | 23.1 | 127.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorovskikh, S.I.; Tryakhov, D.E.; Klyamer, D.D.; Sukhikh, A.S.; Mirzaeva, I.V.; Morozova, N.B.; Basova, T.V. The Theoretical and Experimental Investigation of the Fluorinated Palladium β-Diketonate Derivatives: Structure and Physicochemical Properties. Molecules 2022, 27, 2207. https://doi.org/10.3390/molecules27072207
Dorovskikh SI, Tryakhov DE, Klyamer DD, Sukhikh AS, Mirzaeva IV, Morozova NB, Basova TV. The Theoretical and Experimental Investigation of the Fluorinated Palladium β-Diketonate Derivatives: Structure and Physicochemical Properties. Molecules. 2022; 27(7):2207. https://doi.org/10.3390/molecules27072207
Chicago/Turabian StyleDorovskikh, Svetlana I., Denis E. Tryakhov, Darya D. Klyamer, Alexander S. Sukhikh, Irina V. Mirzaeva, Natalia B. Morozova, and Tamara V. Basova. 2022. "The Theoretical and Experimental Investigation of the Fluorinated Palladium β-Diketonate Derivatives: Structure and Physicochemical Properties" Molecules 27, no. 7: 2207. https://doi.org/10.3390/molecules27072207
APA StyleDorovskikh, S. I., Tryakhov, D. E., Klyamer, D. D., Sukhikh, A. S., Mirzaeva, I. V., Morozova, N. B., & Basova, T. V. (2022). The Theoretical and Experimental Investigation of the Fluorinated Palladium β-Diketonate Derivatives: Structure and Physicochemical Properties. Molecules, 27(7), 2207. https://doi.org/10.3390/molecules27072207