Retusone A, a Guaiane-Type Sesquiterpene Dimer from Wikstroemia retusa and Its Inhibitory Effects on Histone Acetyltransferase HBO1 Expression
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of 1 and 2
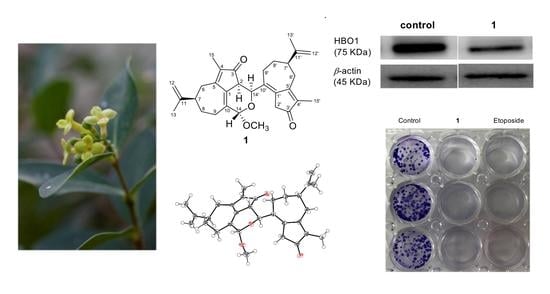
2.2. Inhibitory Effects of 1 on HBO1 Expression and Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Isolation of Compounds
4.4. Structural Elucidation
4.5. Crystallography
4.6. Calculation of the ECD Spectrum
4.7. Cell Culture
4.8. In Vitro Reporter Gene Assay
4.9. Determination of Cell Viability
4.10. The Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-qPCR)
4.11. Western Blot Analysis
4.12. Crystal Violet Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, M.; Stillmann, B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 1999, 274, 23027–23034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, M.; Takahashi, Y.; Mizzen, C.A.; Cook, R.C.; Fujita, M.; Allis, C.D.; Frierson, H.F., Jr.; Fukusato, T.; Smith, M.M. Histone acetyltransferase Hbo1: Catalytic activity, cellular abundance, and links to primary cancers. Gene 2009, 436, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Stern, H.M.; Ge, L.; O’Brien, C.; Haydu, L.; Honchell, C.D.; Haverty, P.M.; Peters, B.A.; Wu, T.D.; Amler, L.C.; et al. Genetic Alterations and Oncogenic Pathways Associated with Breast Cancer Subtypes. Mol. Cancer Res. 2009, 7, 511–522. [Google Scholar]
- Miotto, B.; Struhl, K. HBO1 histone acetylase is a coactivator of the replication licensing factor Cdt1. Genes Dev. 2008, 22, 2633–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, Y.; Miyagi, S.; Saraya, A.; Negish, M.; Endoh, M.; Endo, T.K.; Toyoda, T.; Shinga, J.; Katumoto, T.; Chiba, T.; et al. The Hbo1-Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood 2011, 118, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Kueh, A.J.; Dixon, M.P.; Voss, A.K.; Thomas, T. HBO1 Is required for H3K14 acetylation and normal transcriptional activity during Embryonic Development. Mol. Cell. Biol. 2011, 31, 845–860. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Gilletrr, G.W. Chemotaxonomic sturdies of Hawaiian Wikstroemia. Econ. Bot. 1963, 23, 24–31. [Google Scholar] [CrossRef]
- Lin, R.-W.; Tsai, I.-L.; Duh, C.-Y.; Lee, K.-H.; Chen, I.-S. New lignans and cytotoxic constituents from Wikstroemia lanceolate. Plant Med. 2004, 70, 234–238. [Google Scholar]
- Jiang, H.Z.; Ma, Q.G.; Hyang, S.Z.; Liang, W.J.; Wang, P.C.; Hu, J.M.; Zhou, J.; Ahao, Y.X. A new guaiane-type sesquiterpene with 15 known compouds from Wikstroemia scytophylla Diels. Chin. J. Chem. 2012, 30, 1335–1338. [Google Scholar] [CrossRef]
- Liu, A.H.; Dong, M.G.; Chang, H.; Han, N.; Yin, J. Guaiane type of sesquiterpene with NO inhibitory from the root of Wikstroemia indica. Bioorg. Chem. 2020, 99, 103785. [Google Scholar] [CrossRef] [PubMed]
- Yaga, S.; Kinjo, K.; Hayashi, H.; Matuso, N.; Abe, F.; Yamauchi, T. Diterpenoids with the daphnane skeleton from Wikstroemia retusa. Phytochemistry 1993, 32, 141–143. [Google Scholar]
- Abe, F.; Iwase, Y.; Yamauchi, T.; Kinjo, K.; Yaga, S. Daphnane diterpenoids from the bark of Wikstroemia retusa. Phytochemistry 1997, 44, 643–647. [Google Scholar] [CrossRef]
- Abe, F.; Iwase, Y.; Yamauchi, T.; Kinjo, K.; Yaga, S.; Ishi, M.; Iwahana, M. Minor daphnane-type diterpenoids from Wikstroemia retusa. Phytochemistry 1998, 47, 833–837. [Google Scholar] [CrossRef]
- Wang, N.; Nako, S.; Ueda, K.; Niwa, M. Phenolic constituents of Wikstroemia retusa. Planta Med. 1992, 58, 573. [Google Scholar] [CrossRef]
- Taninaka, H.; Takaishi, Y.; Honda, G.; Imakura, Y.; Sezik, E.; Yesilada, E. Terpenoids and aromatic compounds from Daphne oleoides ssp. oleoides. Phytochemistry 1999, 52, 1525–1529. [Google Scholar] [CrossRef]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef]
- Kato, M.; He, Y.-M.; Dibwe, D.; Li, F.; Awale, S.; Kadota, S.; Tezuka, Y. New guaian-type sesquiterpene from Wikstromia indica. Nat. Prod. Comm. 2013, 9, 1934578X1400900101. [Google Scholar]
- Ingert, N.; Bombarda, I.; Herbette, G.; Faure, R.; Moretti, C.; Raharivelomanana, P. Oleodaphnoic acid and coriaceol, two New Natural products form the stem bark of Wikstromia coriacea. Molecules 2013, 18, 2988–2996. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, L.; Amplue, J.; Yeung, M.M.; Lam, E.Y.N.; Chan, Y.-C.; Weng, C.-F.; Yeh, P.; Knezevic, K.; Butler, M.S.; Hoegi, A.; et al. HBO1 is required for the maintenance of leukaemia stemm cells. Nature 2020, 577, 266–270. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Deng, L.; Chen, G.; Liu, Y. HBO1 overexpression is important for hepatocellular carcinoma cell growth. Cell Death Dis. 2021, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Susa, T.; Takahashi, Y.; Tamamori-Adachi, M.; Kajitani, T.; Okinaga, H.; Fukusato, T.; Okazaki, T. Histone acetyltransferase Hboi destabilized estrogen receptor a by ubiquitination and modulates proliferation of breast cancers. Cancer Sci. 2013, 104, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2020-3: MacroModel, Schrödinger; LLC: New York, NY, USA, 2020.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis Version 1.71, Berlin, Germany, 2017. Available online: http:/specdis-software.jimdo.com (accessed on 10 May 2021).






| Position | δH | δC Type | COSY | HMBC |
|---|---|---|---|---|
| 1 | 133.8, C | |||
| 2 | 3.09, d (10.4) | 47.7, CH | 14′ | 1, 3, 14′ |
| 3 | 200.1, C | |||
| 4 | 140.2, C | |||
| 5 | 163.8, C | |||
| 6 | 2.77, bd (17.8) | 35.7, CH2 | 7 | 1, 5, 7 |
| 7 | 2.57 b | 42.9, CH | 6, 8 | 9, 12, 13 |
| 8 | 1.87 b, 2.00 b | 31.9, CH2 | 7, 9 | 6, 7, 9, 10 |
| 9 | 2.22, ddd (4.2, 9.4, 18.2), 2.63 b | 29.4, CH2 | 8 | 1, 7, 8 |
| 10 | 130.7, C | |||
| 11 | 148.7, C | |||
| 12 | 4.71, bm, 4.77, m | 109.7, CH2 | 13 | 7, 11, 13 |
| 13 | 1.78, s | 20.6, CH3 | 12 | 7, 11, 12 |
| 14 | 4.86, s | 99.2, CH | 1, 9, 10 | |
| 15 | 1.74, s | 8.3, CH3 | 3, 4, 5 | |
| 1′ | 136.6, C | |||
| 2′ | 2.90 b,c | 39.1, CH | 1′, 3′, 5′, 10 | |
| 3′ | 204.6, C | |||
| 4′ | 140.0, C | |||
| 5′ | 167.4, C | |||
| 6′ | 2.84 b,c | 32.5, CH2 | 7′ | 1′, 4′, 5′, 7′, 8′ |
| 7′ | 2.60 b | 42.7, CH | 6′, 8′ | 6′, 8′, 9′ |
| 8′ | 1.94, ddd (2.5, 7.1, 14.1), 2.02 b | 32.3, CH2 | 7′, 9′ | 1′, 4′, 5′, 7′, 8′ |
| 9′ | 2.50, ddd (2.5, 7.6, 16.4), 2.61 b | 24.3, CH2 | 8′ | 1′, 7′, 10′ |
| 10′ | 135.9, C | |||
| 11′ | 149.4, C | |||
| 12′ | 4.75, m, 4.81, bm | 110.2, CH2 | 13′ | 7′, 11′, 13′ |
| 13′ | 1.79, s | 20.7, CH3 | 12′ | 7′, 11′, 12′ |
| 14′ | 4.40, d (10.4) | 69.8, CH | 2′ | 1, 3, 14, 1′, 9′, 10′ |
| 15′ | 1.80, s | 8.4, CH3 | 3′, 4′, 5′ | |
| OCH3 | 3.39, s | 55.7, CH3 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.S.; Nakano, T.; Fukaya, H.; Hitotsuyanagi, Y.; Nakamura, M.; Umetsu, M.; Matsushita, N.; Miyake, K.; Fuchino, H.; Kawahara, N.; et al. Retusone A, a Guaiane-Type Sesquiterpene Dimer from Wikstroemia retusa and Its Inhibitory Effects on Histone Acetyltransferase HBO1 Expression. Molecules 2022, 27, 2909. https://doi.org/10.3390/molecules27092909
Yun YS, Nakano T, Fukaya H, Hitotsuyanagi Y, Nakamura M, Umetsu M, Matsushita N, Miyake K, Fuchino H, Kawahara N, et al. Retusone A, a Guaiane-Type Sesquiterpene Dimer from Wikstroemia retusa and Its Inhibitory Effects on Histone Acetyltransferase HBO1 Expression. Molecules. 2022; 27(9):2909. https://doi.org/10.3390/molecules27092909
Chicago/Turabian StyleYun, Young Sook, Tomomi Nakano, Haruhiko Fukaya, Yukio Hitotsuyanagi, Miho Nakamura, Megumi Umetsu, Nobuko Matsushita, Katsunori Miyake, Hiroyuki Fuchino, Nobuo Kawahara, and et al. 2022. "Retusone A, a Guaiane-Type Sesquiterpene Dimer from Wikstroemia retusa and Its Inhibitory Effects on Histone Acetyltransferase HBO1 Expression" Molecules 27, no. 9: 2909. https://doi.org/10.3390/molecules27092909
APA StyleYun, Y. S., Nakano, T., Fukaya, H., Hitotsuyanagi, Y., Nakamura, M., Umetsu, M., Matsushita, N., Miyake, K., Fuchino, H., Kawahara, N., Moriya, F., Ito, A., Takahashi, Y., & Inoue, H. (2022). Retusone A, a Guaiane-Type Sesquiterpene Dimer from Wikstroemia retusa and Its Inhibitory Effects on Histone Acetyltransferase HBO1 Expression. Molecules, 27(9), 2909. https://doi.org/10.3390/molecules27092909