Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
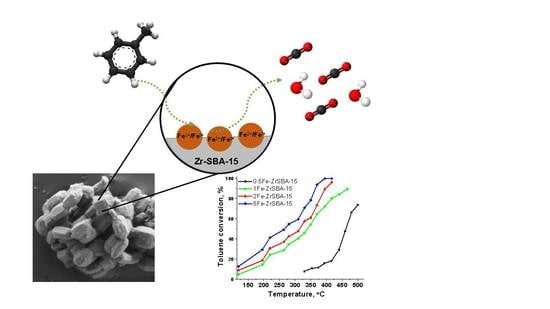
2.2. Toluene Oxidation Catalytic Tests
3. Experimental
3.1. Synthesis of the Catalysts
3.2. Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coggon, M.M.; Gkatzelis, G.I.; McDonald, B.C.; Gilman, J.B.; Schwantes, R.H.; Abuhassan, N.; Aikin, K.C.; Arend, M.F.; Berkoff, T.A.; Brown, S.S.; et al. Volatile Chemical Product Emissions Enhance Ozone and Modulate Urban Chemistry. Proc. Natl. Acad. Sci. USA 2021, 118, e2026653118. [Google Scholar] [CrossRef] [PubMed]
- Albanese, S.; Cicchella, D. Legacy Problems in Urban Geochemistry. Elements 2012, 8, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Li, A.J.; Pal, V.K.; Kannan, K. A Review of Environmental Occurrence, Toxicity, Biotransformation and Biomonitoring of Volatile Organic Compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health BT—Air Pollution and Control; Sharma, N., Agarwal, A.K., Eastwood, P., Gupta, T., Singh, A.P., Eds.; Springer Singapore: Singapore, 2018; pp. 119–142. ISBN 978-981-10-7185-0. [Google Scholar]
- Billionnet, C.; Sherrill, D.; Annesi-Maesano, I. Estimating the Health Effects of Exposure to Multi-Pollutant Mixture. Ann. Epidemiol. 2012, 22, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic Oxidation of Volatile Organic Compounds (VOCs)—A Review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent Advances in VOC Elimination by Catalytic Oxidation Technology onto Various Nanoparticles Catalysts: A Critical Review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous Molecular Sieve-Based Materials for Catalytic Oxidation of VOC: A Review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic Oxidation of Volatile Organic Compounds on Supported Noble Metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Barakat, T.; Rooke, J.C.; Tidahy, H.L.; Hosseini, M.; Cousin, R.; Lamonier, J.-F.; Giraudon, J.-M.; De Weireld, G.; Su, B.-L.; Siffert, S. Noble-Metal-Based Catalysts Supported on Zeolites and Macro-Mesoporous Metal Oxide Supports for the Total Oxidation of Volatile Organic Compounds. ChemSusChem 2011, 4, 1420–1430. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Kim, J.-H.; Kim, J.-H.; Kang, S.-H.; Ryu, J.-H.; Park, N.-K.; Yun, D.-S.; Bae, J.-W. Noble-Metal-Based Catalytic Oxidation Technology Trends for Volatile Organic Compound (VOC) Removal. Catalysts 2022, 12, 63. [Google Scholar] [CrossRef]
- Pineda, A.; Ojeda, M.; Romero, A.A.; Balu, A.M.; Luque, R. Mechanochemical Synthesis of Supported Cobalt Oxide Nanoparticles on Mesoporous Materials as Versatile Bifunctional Catalysts. Microporous Mesoporous Mater. 2018, 272, 129–136. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.-C.; Park, Y.-K. Recent Advances in Volatile Organic Compounds Abatement by Catalysis and Catalytic Hybrid Processes: A Critical Review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic Removal of Volatile Organic Compounds Using Ordered Porous Transition Metal Oxide and Supported Noble Metal Catalysts. Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Murindababisha, D.; Yusuf, A.; Sun, Y.; Wang, C.; Ren, Y.; Lv, J.; Xiao, H.; Chen, G.Z.; He, J. Current Progress on Catalytic Oxidation of Toluene: A Review. Environ. Sci. Pollut. Res. 2021, 28, 62030–62060. [Google Scholar] [CrossRef]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.M.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, Á.; Cherkezova-Zheleva, Z.; Mitov, I.; Kostova, N.; Tsoncheva, T. Toluene Oxidation on Titanium- and Iron-Modified MCM-41 Materials. J. Hazard. Mater. 2009, 168, 226–232. [Google Scholar] [CrossRef]
- Du, Q.; Rao, R.; Bi, F.; Yang, Y.; Zhang, W.; Yang, Y.; Liu, N.; Zhang, X. Preparation of Modified Zirconium-Based Metal-Organic Frameworks (Zr-MOFs) Supported Metals and Recent Application in Environment: A Review and Perspectives. Surf. Interfaces 2022, 28, 101647. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M.; Pilling, M.J. Photochemical Ozone Creation Potentials for Organic Compounds in Northwest Europe Calculated with a Master Chemical Mechanism. Atmos. Environ. 1998, 32, 2429–2441. [Google Scholar] [CrossRef]
- Malakootian, M.; Maleki, S.; Rajabi, S.; Hasanzadeh, F.; Nasiri, A.; Mohammdi, A.; Faraji, M. Source Identification, Spatial Distribution and Ozone Formation Potential of Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) Emissions in Zarand, an Industrial City of Southeastern Ira. J. Air Pollut. Health 2022, 7, 217–232. [Google Scholar] [CrossRef]
- Yue, Y.; Gédéon, A.; Bonardet, J.-L.; D’Espinose, J.-B.; Fraissard, J.; Melosh, N. Direct Synthesis of AlSBA Mesoporous Molecular Sieves: Characterization and Catalytic Activities. Chem. Commun. 1999, 19, 1967–1968. [Google Scholar] [CrossRef]
- Ojeda, M.; Balu, A.M.; Barrón, V.; Pineda, A.; Coleto, Á.G.; Romero, A.Á.; Luque, R. Solventless Mechanochemical Synthesis of Magnetic Functionalized Catalytically Active Mesoporous SBA-15 Nanocomposites. J. Mater. Chem. A 2014, 2, 387–393. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Šuligoj, A.; Štangar, U.L.; Ristić, A.; Mazaj, M.; Verhovšek, D.; Tušar, N.N. TiO2–SiO2 Films from Organic-Free Colloidal TiO2 Anatase Nanoparticles as Photocatalyst for Removal of Volatile Organic Compounds from Indoor Air. Appl. Catal. B Environ. 2016, 184, 119–131. [Google Scholar] [CrossRef]
- Lukens, W.W.; Schmidt-Winkel, P.; Zhao, D.; Feng, J.; Stucky, G.D. Evaluating Pore Sizes in Mesoporous Materials: A Simplified Standard Adsorption Method and a Simplified Broekhoff-de Boer Method. Langmuir 1999, 15, 5403–5409. [Google Scholar] [CrossRef]
- Tanev, P.T.; Vlaev, L.T. An Attempt at a More Precise Evaluation of the Approach to Mesopore Size Distribution Calculations Depending on the Degree of Pore Blocking. J. Colloid Interface Sci. 1993, 160, 110–116. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, Á.; Lázár, K.; Károly, Z. The Physico-Chemical and Catalytic Properties of Ferrite-Containing MCM-41 and SBA-15 Materials. Microporous Mesoporous Mater. 2012, 151, 180–187. [Google Scholar] [CrossRef]
- Taylor, S.H.; Heneghan, C.S.; Hutchings, G.J.; Hudson, I.D. The Activity and Mechanism of Uranium Oxide Catalysts for the Oxidative Destruction of Volatile Organic Compounds. Catal. Today 2000, 59, 249–259. [Google Scholar] [CrossRef]
- Minicò, S.; Scirè, S.; Crisafulli, C.; Maggiore, R.; Galvagno, S. Catalytic Combustion of Volatile Organic Compounds on Gold/Iron Oxide Catalysts. Appl. Catal. B Environ. 2000, 28, 245–251. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lee, J.-F.; Cheng, S. Pinacol-Type Rearrangement Catalyzed by Zr-Incorporated SBA-15. J. Catal. 2010, 270, 196–205. [Google Scholar] [CrossRef]







| Sample\Element | Fe (wt.%) | Al (wt.%) | Zr (wt.%) | SBET (m2/g) | Vtot (cm3/g) | WBJH (nm) |
|---|---|---|---|---|---|---|
| SBA-15 | - | - | - | 563 | 0.833 | 7.1 |
| 0.5Fe-SBA-15 | 0.47 | - | - | 361 | 0.452 | 7.0 |
| 1Fe-SBA-15 | 1.02 | - | - | 366 | 0.482 | 7.1 |
| 2Fe-SBA-15 | 2.11 | - | - | 352 | 0.458 | 7.2 |
| 5Fe-SBA-15 | 4.86 | - | - | 400 | 0.479 | 6.8 |
| Al-SBA-15 | - | 1.43 | - | 712 | 1.011 | 8.7 |
| 0.5Fe-AlSBA-15 | 0.41 | 1.57 | - | 447 | 0.600 | 8.8 |
| 1Fe-AlSBA-15 | 1.00 | 1.64 | - | 415 | 0.542 | 8.9 |
| 2Fe-AlSBA-15 | 2.11 | 1.38 | - | 395 | 0.504 | 8.8 |
| 5Fe-AlSBA-15 | 4.77 | 1.29 | - | 422 | 0.482 | 8.4 |
| Zr-SBA-15 | - | - | 4.77 | 759 | 0.891 | 7.9 |
| 0.5Fe-ZrSBA-15 | 0.48 | - | 4.25 | 378 | 0.427 | 7.9 |
| 1Fe-ZrSBA-15 | 1.05 | - | 4.84 | 363 | 0.414 | 7.8 |
| 2Fe-ZrSBA-15 | 2.07 | - | 4.58 | 382 | 0.411 | 7.7 |
| 5Fe-ZrSBA-15 | 4.94 | - | 4.47 | 467 | 0.452 | 7.7 |
| Samples | O (at.%) | Si (at.%) | Fe (at.%) | Zr (at.%) | Al (at.%) |
|---|---|---|---|---|---|
| 5Fe-ZrSBA-15 | 61.8 | 36.5 | 0.8 | 0.9 | - |
| Spent 5Fe-ZrSBA-15 | 64.4 | 34.1 | 0.7 | 0.8 | - |
| 5Fe-SBA-15 | 63.1 | 36.5 | 0.4 | - | - |
| 5Fe-AlSBA-15 | 63.6 | 35.3 | 0.3 | - | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, I.; Ojeda, M.; Andresen, J.M.; Ristić, A.; Dimitrov, M.; Tušar, N.N.; Atanasova, G.; Popova, M. Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials. Molecules 2023, 28, 204. https://doi.org/10.3390/molecules28010204
Trendafilova I, Ojeda M, Andresen JM, Ristić A, Dimitrov M, Tušar NN, Atanasova G, Popova M. Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials. Molecules. 2023; 28(1):204. https://doi.org/10.3390/molecules28010204
Chicago/Turabian StyleTrendafilova, Ivalina, Manuel Ojeda, John M. Andresen, Alenka Ristić, Momtchil Dimitrov, Nataša Novak Tušar, Genoveva Atanasova, and Margarita Popova. 2023. "Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials" Molecules 28, no. 1: 204. https://doi.org/10.3390/molecules28010204
APA StyleTrendafilova, I., Ojeda, M., Andresen, J. M., Ristić, A., Dimitrov, M., Tušar, N. N., Atanasova, G., & Popova, M. (2023). Low-Temperature Toluene Oxidation on Fe-Containing Modified SBA-15 Materials. Molecules, 28(1), 204. https://doi.org/10.3390/molecules28010204