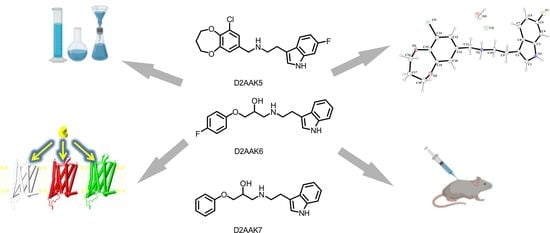
Synthesis, Structural and Behavioral Studies of Indole Derivatives D2AAK5, D2AAK6 and D2AAK7 as Serotonin 5-HT1A and 5-HT2A Receptor Ligands
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. X-ray Studies of D2AAK5
2.3. Molecular Modeling
2.4. Behavioral Studies
2.4.1. Spontaneous Locomotor Activity
2.4.2. Motor Coordination
2.4.3. Effect of Acute Administration of D2AAK5, D2AAK6 (7.5 mg/kg) and D2AAK7 (4 and 2 mg/kg) on Elevated Plus-Maze (EPM) Performance in Mice
2.4.4. Effect of Acute Administration of D2AAK5, D2AAK6 (7.5 mg/kg), and D2AAK7 (4 mg/kg) on the Total Duration of Immobility in the Forced Swim Test (FST) in Mice
2.4.5. Effect of Acute Administration of D2AAK5, D2AAK6 (7.5 mg/kg) and D2AAK7 (4 mg/kg) on Memory Consolidation in Passive Avoidance (PA) Test in Mice
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General
4.1.2. Synthesis of 5-Chlorovanillin
4.1.3. Synthesis of 3-Chloro-4,5-dihydroxybenzaldehyde
4.1.4. Synthesis of 9-Chloro-3,4-dihydro-2H-benzo[b][1,4]dioxepine-7-carbaldehyde 4
4.1.5. Synthesis of 6-Fluoro-1H-indole-3-carbaldehyde
4.1.6. Synthesis of 6-Fluoro-3-(2-nitrovinyl)-1H-indole
4.1.7. Synthesis of 6-Fluorotryptamine 5
4.1.8. Synthesis of N-((9-Chloro-3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-yl)methyl)-2-(6-fluoro-1H-indol-3-yl)ethan-1-amine 1 (D2AAK5)
4.1.9. Synthesis of 1-((2-(1H-Indol-3-yl)ethyl)amino)-3-(4-fluorophenoxy)propan-2-ol 2 (D2AAK6)
4.1.10. Synthesis of 1-((2-(1H-Indol-3-yl)ethyl)amino)-3-phenoxypropan-2-ol 3 (D2AAK7)
4.2. X-ray Studies
4.3. Molecular Modeling
4.3.1. Ligand Preparation
4.3.2. Protein Preparation
4.3.3. Molecular Docking and MM/GBSA Calculations
4.3.4. Molecular Dynamics Simulations
4.4. Behavioral Studies
4.4.1. General Procedures
4.4.2. Spontaneous Locomotor Activity
4.4.3. Motor Coordination
4.4.4. EPM Test
4.4.5. FST in Mice
4.4.6. PA Task
4.4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sriram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Stackman, R.W. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. Overcoming depression with 5-HT2A receptor ligands. Int. J. Mol. Sci. 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Silva, A.G.; Loza, M.I.; Kolb, P.; Castro, M.; Poso, A. Structure-based virtual screening for dopamine D2 receptor ligands as potential antipsychotics. ChemMedChem 2016, 11, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Targowska-Duda, K.M.; Budzyńska, B.; Biała, G.; Silva, A.G.; Castro, M. In Vitro, molecular modeling and behavioral studies of 3-{[4-(5-Methoxy-1H-Indol-3-Yl)-1,2,3,6-Tetrahydropyridin-1-Yl]Methyl}-1,2-Dihydroquinolin-2-One (D2AAK1) as a Potential Antipsychotic. Neurochem. Int. 2016, 96, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Kondej, M.; Wróbel, T.M.; Silva, A.G.; Stępnicki, P.; Koszła, O.; Kędzierska, E.; Bartyzel, A.; Biała, G.; Matosiuk, D.; Loza, M.I.; et al. Synthesis, pharmacological and structural studies of 5-substituted-3-(1-Arylmethyl-1,2,3,6-Tetrahydropyridin-4-Yl)-1H-indoles as multi-target ligands of aminergic GPCRs. Eur. J. Med. Chem. 2019, 180, 673–689. [Google Scholar] [CrossRef]
- Kondej, M.; Wróbel, T.M.; Targowska-Duda, K.M.; Leandro Martínez, A.; Koszła, O.; Stępnicki, P.; Zięba, A.; Paz, A.; Wronikowska-Denysiuk, O.; Loza, M.I.; et al. Multitarget derivatives of D2AAK1 as potential antipsychotics: The effect of substitution in the indole moiety. ChemMedChem 2022, 17, e202200238. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Targowska-Duda, K.M.; Stępnicki, P.; Silva, A.G.; Koszła, O.; Kędzierska, E.; Grudzińska, A.; Kruk-Słomka, M.; Biała, G.; Castro, M. N-(3-{4-[3-(Trifluoromethyl)Phenyl]Piperazin-1-Yl}propyl)-1H-Indazole-3-carboxamide (D2AAK3) as a potential antipsychotic: In vitro, in silico and in vivo evaluation of a multi-target ligand. Neurochem. Int. 2021, 146, 105016. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Targowska-Duda, K.M.; Silva, A.G.; Kondej, M.; Biała, G.; Castro, M. N-(2-Hydroxyphenyl)-1-[3-(2-Oxo-2,3-Dihydro-1H- Benzimidazol-1-Yl)Propyl]Piperidine-4-Carboxamide (D2AAK4), a multi-target ligand of aminergic GPCRs, as a potential antipsychotic. Biomolecules 2020, 10, E349. [Google Scholar] [CrossRef]
- Skoreński, M.; Sieńczyk, M. The fellowship of privileged scaffolds-one structure to inhibit them all. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef]
- Nilchan, N.; Phetsang, W.; Nowwarat, T.; Chaturongakul, S.; Jiarpinitnun, C. Halogenated trimethoprim derivatives as multidrug-resistant staphylococcus aureus therapeutics. Bioorg. Med. Chem. 2018, 26, 5343–5348. [Google Scholar] [CrossRef]
- Lange, R.G. Cleavage of Alkyl O-hydroxyphenyl ethers. J. Org. Chem. 1962, 27, 2037–2039. [Google Scholar] [CrossRef]
- Pessoa-Mahana, H.; Silva-Matus, P.; Pessoa-Mahana, C.D.; Chung, H.; Iturriaga-Vásquez, P.; Quiroz, G.; Möller-Acuña, P.; Zapata-Torres, G.; Saitz-Barría, C.; Araya-Maturana, R.; et al. Synthesis and docking of novel 3-indolylpropyl derivatives as new polypharmacological agents displaying affinity for 5-HT1AR/SERT. Arch. Pharm. 2017, 350, e1600271. [Google Scholar] [CrossRef] [PubMed]
- Ono, N. The Nitro-Aldol (Henry) reaction. In The Nitro Group in Organic Synthesis; John Wiley & Sons, Ltd.: NewYork, NY, USA, 2001; pp. 30–69. ISBN 978-0-471-22448-8. [Google Scholar]
- Zhao, X.; Zhou, Y.; Li, B.-L.; Du, G.; Yu, Z. Highly diastereoselective cascade dearomatization of 3-(2-Isocyanoethyl)indoles with nitrile imines: A facile access to unexpected polycyclic indolines. Org. Chem. Front. 2022, 9, 1336–1342. [Google Scholar] [CrossRef]
- Kondej, M.; Bartyzel, A.; Pitucha, M.; Wróbel, T.M.; Silva, A.G.; Matosiuk, D.; Castro, M.; Kaczor, A.A. Synthesis, structural and thermal studies of 3-(1-Benzyl-1,2,3,6-Tetrahydropyridin-4-Yl)-5-Ethoxy-1H-Indole (D2AAK1_3) as dopamine D₂ receptor ligand. Molecules 2018, 23, E2249. [Google Scholar] [CrossRef] [Green Version]
- Bartyzel, A.; Kondej, M.; Stępnicki, P.; Wróbel, T.M.; Kaczor, A.A. Experimental and computational structural studies of 5-substituted-3-(1-Arylmethyl-1,2,3,6-Tetrahydropyridin-4-Yl)-1H-Indoles. J. Mol. Struct. 2021, 1245, 130998. [Google Scholar] [CrossRef]
- Bartyzel, A.; Kaczor, A.A.; Mahmoudi, G.; Masoudiasl, A.; Wróbel, T.M.; Pitucha, M.; Matosiuk, D. Experimental and computational structural studies of 2,3,5-Trisubstituted and 1,2,3,5-tetrasubstituted indoles as non-competitive antagonists of GluK1/GluK2 receptors. Molecules 2022, 27, 2479. [Google Scholar] [CrossRef]
- Boessenkool, I.K.; Boeyens, J.C.A. Identification of the conformational type of seven-membered rings. J. Cryst. Mol. Struct. 1980, 10, 11–18. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Bartuzi, D.; Kaczor, A.A.; Targowska-Duda, K.M.; Matosiuk, D. Recent advances and applications of molecular docking to G protein-coupled receptors. Molecules 2017, 22, E340. [Google Scholar] [CrossRef] [Green Version]
- Kaczor, A.A.; Rutkowska, E.; Bartuzi, D.; Targowska-Duda, K.M.; Matosiuk, D.; Selent, J. Computational methods for studying g protein-coupled receptors (GPCRs). Methods Cell Biol. 2016, 132, 359–399. [Google Scholar] [CrossRef]
- Bueschbell, B.; Barreto, C.A.V.; Preto, A.J.; Schiedel, A.C.; Moreira, I.S. A complete assessment of dopamine receptor—Ligand interactions through computational methods. Molecules 2019, 24, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magli, E.; Kędzierska, E.; Kaczor, A.A.; Bielenica, A.; Severino, B.; Gibuła-Tarłowska, E.; Kotlińska, J.H.; Corvino, A.; Sparaco, R.; Esposito, G.; et al. Synthesis, docking studies, and pharmacological evaluation of 2-Hydroxypropyl-4-Arylpiperazine derivatives as serotoninergic ligands. Arch. Pharm. 2021, 354, e2000414. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Chapter 9.5 Typical interatomic distances: Organic compounds in international tables for crystallography. In International Tables for Crystallography Vol. C; John Wiley & Sons, Ltd.: NewYork, NY, USA, 2006. [Google Scholar]
- Rasztawicka, M.; Wolska, I.; Maciejewska, D. Solid State Structure by X-ray and 13C CP/MAS NMR of New 5,5′-Diethoxy-3,3′-Methanediyl-Bis-Indole. J. Mol. Struct. 2007, 831, 174–179. [Google Scholar] [CrossRef]
- Chandrakantha, T.N.; Puttaraja, P.; Kokila, M.K.; Shivaprakash, N.C. Ethyl 5-Ethoxy-3-Methyl-1H-Indole-2-Carboxylate. Acta Cryst. C 1998, 54, 1685–1687. [Google Scholar] [CrossRef]
- Rusew, R.; Kurteva, V.; Shivachev, B. Novel quaternary ammonium derivatives of 4-Pyrrolidino Pyridine: Synthesis, structural, thermal, and antibacterial studies. Crystals 2020, 10, 339. [Google Scholar] [CrossRef]
- Yasutake, M.; Yamaguchi, S.; Hirose, T. Crystal Structure of 3,4-Dihydro-2H-1,5-Benzodioxepine-7,8-Dicarboxylic Acid. Anal. Sci: X-ray Struct. Anal. Online 2005, 21, x81–x82. [Google Scholar] [CrossRef] [Green Version]
- Kraft, P.; Popaj, K.; Müller, P.; Schär, M. ‘Vanilla Oceanics’: Synthesis and olfactory properties of (1′E)-7-(Prop-1′-Enyl)-2H-Benzo[b][1,4]Dioxepin-3(4H)-ones and homologues. Synthesis 2010, 2010, 3029–3036. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays; Vogel, H., Ed.; Springer: Berlin Heidelberg, Germany, 2008; p. 565. [Google Scholar]
- Ari, C.; D’Agostino, D.P.; Diamond, D.M.; Kindy, M.; Park, C.; Kovács, Z. Elevated plus maze test combined with video tracking software to investigate the anxiolytic effect of exogenous ketogenic supplements. J. Vis. Exp. 2019, 140, 10. [Google Scholar] [CrossRef]
- Howland, R.H. Buspirone: Back to the future. J. Psychosoc. Nurs. Ment. Health. Serv. 2015, 53, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Marciniak, M. Ligandy receptora 5-HT1A jako potencjalne leki przeciwdepresyjne. Biul. Wydz. Farm. WUM 2015, 5, 28–39. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of anxiety disorders: Current and emerging treatment options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef] [PubMed]
- Khouzam, H.R. A review of trazodone use in psychiatric and medical conditions. Postgrad. Med. 2017, 129, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Jarosz, J.; Wasik, A.; Jastrzębska-Więsek, M.; Zagórska, A.; Pawłowski, M.; Wesołowska, A. Novel Tricyclic [2,1-f]Theophylline derivatives of LCAP with activity in mouse models of affective disorders. J. Pharm. Pharmacol. 2014, 66, 1755–1762. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Christensen, M.C.; Loft, H.; Florea, I.; McIntyre, R.S. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019, 24, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Verdurand, M.; Zimmer, L. Hippocampal 5-HT1A receptor expression changes in prodromal stages of alzheimer’s disease: Beneficial or deleterious? Neuropharmacology 2017, 123, 446–454. [Google Scholar] [CrossRef]
- Agilent Technologies Ltd. CrysAlis PRO; Agilent Technologies Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst.. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger Release 2019-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2019.
- Schrödinger Release 2019-4: Epik; Schrödinger, LLC: New York, NY, USA, 2019.
- Kimura, K.T.; Asada, H.; Inoue, A.; Kadji, F.M.N.; Im, D.; Mori, C.; Arakawa, T.; Hirata, K.; Nomura, Y.; Nomura, N.; et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat. Struct. Mol. Biol. 2019, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2019-4: BioLuminate; Schrödinger, LLC: New York, NY, USA, 2019.
- Ozvoldik, K.; Stockner, T.; Rammner, B.; Krieger, E. Assembly of biomolecular gigastructures and visualization with the vulkan graphics API. J. Chem. Inf. Model. 2021, 61, 5293–5303. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G Protein-coupled receptors. In Methods in Neurosciences; Sealfon, S.C., Ed.; Receptor Molecular Biology; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2022.
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Bowers Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.I.; Kolossváry, I.; Moraes, M.A.; Sacerdoti, F.A.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing (SC06), Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Gross, F.; Tripod, J.; Meier, R. Pharmacological characteristics of the soporific doriden. Schweiz. Med. Wochensch. 1955, 85, 305–309. [Google Scholar]
- Boissier, J.-R.; Tardy, J.; Diverres, J.-C. Une Nouvelle Méthode Simple Pour Explorer l’action «tranquillisante»: Le Test de La Cheminée. PHA 1960, 3, 81–84. [Google Scholar] [CrossRef]
- Lister, R.G. The Use of a Plus-Maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Venault, P.; Chapouthier, G.; de Carvalho, L.P.; Simiand, J.; Morre, M.; Dodd, R.H.; Rossier, J. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature 1986, 321, 864–866. [Google Scholar] [CrossRef] [PubMed]














| Formula | C20H23Cl2FN2O3 |
|---|---|
| Formula weight | 429.30 |
| Temperature K | 120(2) |
| Crystal system | triclinic |
| Space group | P |
| a (Å) | 8.0886(9) |
| b (Å) | 9.0019(7) |
| c (Å) | 14.9140(15) |
| α (°) | 82.422(7) |
| β (°) | 83.707(9) |
| γ (°) | 65.926(9) |
| Volume (Å3) | 980.9(2) |
| Z | 2 |
| Calculated density (g cm−3) | 1.453 |
| μ (mm−1) | 0.365 |
| Absorption correction | multi-scan |
| F(000) | 448 |
| Crystal size (mm) | 0.40 × 0.30 × 0.20 |
| θ range (°) | 2.490 to 26.727 |
| Limiting indices | −8 ≤ h ≤ 10, −7 ≤ k ≤ 11, −18 ≤ l ≤ 18 |
| Reflections collected/unique | 7548/4168 |
| Rint | 0.0436 |
| Data/restraints/parameters | 4168/0/261 |
| GooF (F2) | 1.041 |
| Final R indices [I > 2σ(I)] | R1 = 0.0547, wR2 = 0.1055 |
| R indices (all data) | R1 = 0.0927, wR2 = 0.1241 |
| Largest diff. peak/hole, e Å−3 | 0.356/−0.289 |
| CCDC No. | 2223764 |
| 5-HT1A Receptor | 5-HT2A Receptor | |||
|---|---|---|---|---|
| Ligand | Ki [nM] [5] | MM/GBSA dG Bind kcal/mol | Ki [nM] [5] | MM/GBSA dG Bind kcal/mol |
| D2AAK5 | 938 ± 41 | −40.38 | 135 ± 81 | −52.27 |
| D2AAK6 | 115 ± 29 | −61.03 | 246 ± 99 | −67.91 |
| D2AAK7 | 88.5 ± 14.4 | −48.89 | 546 ± 194 | −65.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczor, A.A.; Kędzierska, E.; Wróbel, T.M.; Grudzińska, A.; Pawlak, A.; Laitinen, T.; Bartyzel, A. Synthesis, Structural and Behavioral Studies of Indole Derivatives D2AAK5, D2AAK6 and D2AAK7 as Serotonin 5-HT1A and 5-HT2A Receptor Ligands. Molecules 2023, 28, 383. https://doi.org/10.3390/molecules28010383
Kaczor AA, Kędzierska E, Wróbel TM, Grudzińska A, Pawlak A, Laitinen T, Bartyzel A. Synthesis, Structural and Behavioral Studies of Indole Derivatives D2AAK5, D2AAK6 and D2AAK7 as Serotonin 5-HT1A and 5-HT2A Receptor Ligands. Molecules. 2023; 28(1):383. https://doi.org/10.3390/molecules28010383
Chicago/Turabian StyleKaczor, Agnieszka A., Ewa Kędzierska, Tomasz M. Wróbel, Angelika Grudzińska, Angelika Pawlak, Tuomo Laitinen, and Agata Bartyzel. 2023. "Synthesis, Structural and Behavioral Studies of Indole Derivatives D2AAK5, D2AAK6 and D2AAK7 as Serotonin 5-HT1A and 5-HT2A Receptor Ligands" Molecules 28, no. 1: 383. https://doi.org/10.3390/molecules28010383
APA StyleKaczor, A. A., Kędzierska, E., Wróbel, T. M., Grudzińska, A., Pawlak, A., Laitinen, T., & Bartyzel, A. (2023). Synthesis, Structural and Behavioral Studies of Indole Derivatives D2AAK5, D2AAK6 and D2AAK7 as Serotonin 5-HT1A and 5-HT2A Receptor Ligands. Molecules, 28(1), 383. https://doi.org/10.3390/molecules28010383