High Hydrostatic Pressure in the Modulation of Enzymatic and Organocatalysis and Life under Pressure: A Review
Abstract
:1. Introduction
2. Effect of HHP on Enzymatic Systems
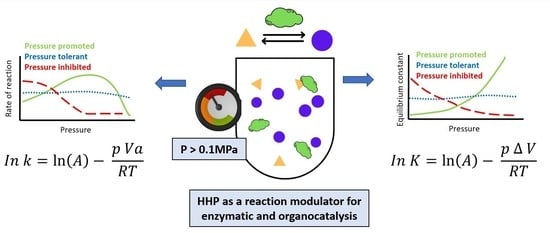
2.1. Effect of HHP on Thermodynamics
2.2. Effects of HHP on Kinetics
3. Effect of High Pressure on the Activity of Particular Enzymes
3.1. Alcohol Dehydrogenase (EC 1.1.1.1)
3.2. Formate Dehydrogenase (EC 1.2.2.1)
3.3. Octopine Dehydrogenase (EC 1.5.1.11)
3.4. Pectin Methylesterase (EC 3.1.1.11)
3.5. β-Glucanase (EC 3.2.1.2)
3.6. Cellulase (EC 3.2.1.4)
3.7. Naringinase (EC 3.2.1.40)
3.8. Chymotrypsin (EC 3.4.21.1)
3.9. Trypsin (EC 3.4.21.4)
3.10. Thermolysin (EC 3.4.24.27)
4. Organocatalysis under Pressure
5. Life’s Response to High Hydrostatic Pressure Environments
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Markets, M. and Enzymes Market Global Forecast. Available online: https://www.marketsandmarkets.com/Market-Reports/enzyme-market-46202020.html (accessed on 24 January 2023).
- Factors, F. Global Food Enzymes Market. Available online: https://www.globenewswire.com/en/news-release/2022/10/05/2529029/0/en/At-6-2-CAGR-Global-Food-Enzymes-Market-Size-to-Surpass-USD-3-104-8-Million-by-2028-Food-Enzymes-Industry-Trends-Value-Analysis-Forecast-by-Facts-Factors.html (accessed on 24 January 2023).
- Gomes, J.; Steiner, W. The Biocatalytic Potential of Extremophiles and Extremozymes. Food Technol. Biotechnol. 2004, 42, 223–235. [Google Scholar]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzym. Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Buckow, R.; Weiss, U.; Heinz, V.; Knorr, D. Stability and Catalytic Activity of α-Amylase from Barley Malt at Different Pressure—Temperature Conditions. Biotechnol. Bioeng. 2006, 97, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.; Nelson, C.M.; Ludlow, J.M.; Shah, N.N.; Clark, D.S. High pressure-temperature bioreactor: Assays of thermostable hydrogenase with fiber optics. Biotechnol. Bioeng. 1988, 34, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Mozhaev, V.V.; Lange, R.; Kudryashova, E.V.; Balny, C. Application of High Hydrostatic Pressure for Increasing Activity and Stability of Enzymes. Biotechnol. Bioeng. 1996, 52, 320–331. [Google Scholar] [CrossRef]
- Michels, P.C.; Hei, D.; Clark, D.S. Pressure Effects on Enzyme Activity and Stability at High Temperatures. Adv. Protein Chem. 1996, 48, 341–376. [Google Scholar] [CrossRef]
- Northrop, D.B. Effects of high pressure on enzymatic activity. Biochim. Biophys. Acta 2002, 1595, 71–79. [Google Scholar] [CrossRef]
- Laidler, K.H. The Influence of Pressure on the Rates of Biological Reactions. Arch. Biochem. 1950, 30, 226–240. [Google Scholar]
- Morild, E. The Theory of Pressure Effects on Enzymes. Adv. Protein Chem. 1981, 34, 93–163. [Google Scholar]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the effects of high hydrostatic pressure in biotechnological applications. Trends Biotechnol. 1994, 12, 493–501. [Google Scholar] [CrossRef]
- Rivalain, N.; Roquain, J.; Demazeau, G. Development of high hydrostatic pressure in biosciences: Pressure effect on biological structures and potential applications in Biotechnologies. Biotechnol. Adv. 2010, 28, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Indrawati; Van Loey, A.; Fachin, D.; Nguyen, B.; Verlent, I.; Hendrickx, M. Overview: Effect of High Pressure on Enzymes Related to Food Quality—Kinetics as a Basis for Process Engineering. High Press. Res. 2002, 22, 613–618. [Google Scholar] [CrossRef]
- Hilz, H.; Lille, M.; Poutanen, K.; Schols, H.A.; Voragen, A.G.J. Combined Enzymatic and High-Pressure Processing Affect Cell Wall Polysaccharides in Berries. J. Agric. Food Chem. 2006, 54, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.; Janssen, A.; Boom, R. Equilibrium shifts in enzyme reactions at high pressure. J. Mol. Catal. B Enzym. 2006, 39, 124–127. [Google Scholar] [CrossRef]
- Kunugi, S.; Kitayaki, M.; Yanagi, Y.; Tanaka, N.; Lange, R.; Balny, C. The Effect of High Pressure on Thermolysin. JBIC J. Biol. Inorg. Chem. 1997, 248, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Krištofíková, D.; Modrocká, V.; Mečiarová, M.; Šebesta, R. Green Asymmetric Organocatalysis. Chemsuschem 2020, 13, 2828–2858. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Lam, Y.-H.; Simon, A.; Houk, K.N. Organocatalysis: Fundamentals and Comparisons to Metal and Enzyme Catalysis. Catalysts 2016, 6, 128. [Google Scholar] [CrossRef]
- Scharf, M.J.; List, B. A Catalytic Asymmetric Pictet–Spengler Platform as a Biomimetic Diversification Strategy toward Naturally Occurring Alkaloids. J. Am. Chem. Soc. 2022, 144, 15451–15456. [Google Scholar] [CrossRef]
- Groβ, M.; Auerbach, G.; Jaenicke, R. The catalytic activities of monomeric enzymes show complex pressure dependence. Fed. Eur. Biochem. Soc. 1993, 321, 256–260. [Google Scholar] [CrossRef]
- Kudryashova, E.V.; Mozhaev, V.V.; Balny, C. Catalytic activity of thermolysin under extremes of pressure and temperature: Modulation by metal ions. Biochim. Biophys. Acta 1998, 1386, 199–210. [Google Scholar] [CrossRef]
- Dallet, S.; Legoy, M.-D. Hydrostatic pressure induces conformational and catalytic changes on two alcohol dehydrogenases but no oligomeric dissociation. Biochim. Biophys. Acta 1996, 1294, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.-S.; Chung, H.-J. Effect of high hydrostatic pressure on the enzyme activities in Saccharomyces cerevisiae and Escherichia coli. New Biotechnol. 2010, 27, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Manera, A.P.; Kuhn, G.; Polloni, A.; Marangoni, M.; Zabot, G.; Kalil, S.J.; de Oliveira, D.; Treichel, H.; Oliveira, J.V.; Mazutti, M.A.; et al. Effect of compressed fluids treatment on the activity, stability and enzymatic reaction performance of β-galactosidase. Food Chem. 2011, 125, 1235–1240. [Google Scholar] [CrossRef]
- Cano, M.P.; Hernandez, A.; Ancos, B. High Pressure and Temperature Effects on Enzyme Inactivation in Strawberry and Orange Products. J. Food Sci. 1997, 62, 85–88. [Google Scholar] [CrossRef]
- Hendrickx, M.; Ludikhuyze, L.; Broeck, I.V.D.; Weemaes, C. Effects of high pressure on enzymes related to food quality. Trends Food Sci. Technol. 1998, 9, 197–203. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Van Loey, A.; Indrawati; Smout, C.; Hendrickx, M.E. Effects of Combined Pressure and Temperature on Enzymes Related to Quality of Fruits and Vegetables: From Kinetic Information to Process Engineering Aspects. Crit. Rev. Food Sci. Nutr. 2003, 43, 527–586. [Google Scholar] [CrossRef]
- Ravash, N.; Peighambardoust, S.H.; Soltanzadeh, M.; Pateiro, M.; Lorenzo, J.M. Impact of high-pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2888–2908. [Google Scholar] [CrossRef]
- Ramirez, R.; Saraiva, J.; Lamela, C.P.; Torres, J.A. Reaction Kinetics Analysis of Chemical Changes in Pressure-Assisted Thermal Processing. Food Eng. Rev. 2009, 1, 16–30. [Google Scholar] [CrossRef]
- Ohmae, E.; Murakami, C.; Gekko, K.; Kato, C. Pressure Effects on Enzyme Functions. J. Biol. Macromol. 2007, 7, 23–29. [Google Scholar]
- Hummer, G.; Garde, S.; García, A.E.; Paulaitis, M.E.; Pratt, L.R. The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 1552–1555. [Google Scholar] [CrossRef]
- Heinz, V.; Buckow, R.; Knorr, D. Catalytic Activity of β-Amylase from Barley in Different Pressure/Temperature Domains. Biotechnol. Prog. 2005, 21, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Yaldagard, M.; Mortazavi, S.A.; Tabatabaie, F. The Principles of Ultra High Pressure Technology and Its Application in Food Processing/ Preservation: A Review of Microbiological and Quality Aspects. Afr. J. Biotechnol. 2008, 7, 2739–2767. [Google Scholar]
- Aertsen, A.; Meersman, F.; Hendrickx, M.E.; Vogel, R.F.; Michiels, C.W. Biotechnology under high pressure: Applications and implications. Trends Biotechnol. 2009, 27, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Vila-Real, H.; Alfaia, A.J.; Phillips, R.S.; Calado, A.R.; Ribeiro, M.H. Pressure-enhanced activity and stability of α-l-rhamnosidase and β-d-glucosidase activities expressed by naringinase. J. Mol. Catal. B Enzym. 2010, 65, 102–109. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. (Eds.) Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Salvador, C.; Santos, M.D.C.; Saraiva, J.A. Effect of the ionic liquid [bmim]Cl and high pressure on the activity of cellulase. Green Chem. 2010, 12, 632–635. [Google Scholar] [CrossRef]
- Muller, P. Glossary of Terms Used in Physical Organic Chemistry. Pure Appl. Chem. 2013, 66, 1077–1184. [Google Scholar] [CrossRef]
- Men, L.; Wang, Y. The Oxidation of Yeast Alcohol Dehydrogenase-1 by Hydrogen Peroxide in Vitro Research. J. Proteone Res. 2007, 6, 216–225. [Google Scholar] [CrossRef]
- Vittorini, M.; Dumitriu, E.; Barletta, G.; Secundo, F. Immobilization of Thermoanaerobium brockii alcohol dehydrogenase on SBA-15. Bioprocess Biosyst. Eng. 2011, 34, 247–251. [Google Scholar] [CrossRef]
- Patel, J.M.; Phillips, R.S. Effects of Hydrostatic Pressure on Stereospecificity of Secondary Alcohol Dehydrogenase from Thermoanaerobacter Ethanolicus Support the Role of Solvation in Enantiospecificity. ACS Catal. 2014, 4, 692–694. [Google Scholar] [CrossRef]
- Jaworek, M.W.; Gajardo-Parra, N.F.; Sadowski, G.; Winter, R.; Held, C. Boosting the kinetic efficiency of formate dehydrogenase by combining the effects of temperature, high pressure and co-solvent mixtures. Colloids Surf. B Biointerfaces 2021, 208, 112127. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.H.; Mueller, A.; Schmitt, L.; Grieshaber, M.K. A Structural Basis for Substrate Selectivity and Stereoselectivity in Octopine Dehydrogenase from Pecten maximus. J. Mol. Biol. 2008, 381, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Jolie, R.P.; Christiaens, S.; De Roeck, A.; Fraeye, I.; Houben, K.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Pectin conversions under high pressure: Implications for the structure-related quality characteristics of plant-based foods. Trends Food Sci. Technol. 2012, 24, 103–118. [Google Scholar] [CrossRef]
- Duvetter, T.; Fraeye, I.; Sila, D.N.; Verlent, I.; Smout, C.; Clynen, E.; Schoofs, L.; Schols, H.; Hendrickx, M.; Van Loey, A. Effect of Temperature and High Pressure on the Activity and Mode of Action of Fungal Pectin Methyl Esterase. Biotechnol. Prog. 2006, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Van Loey, A.; Saraiva, J.A.; Smout, C.; Hendrickx, M. Activity and Process Stability of Purified Green Pepper (Capsicum annuum) Pectin Methylesterase. J. Agric. Food Chem. 2004, 52, 5724–5729. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Satara, Y.; Truong, V.; Van Loey, A.; Hendrickx, M. Combined thermal and high pressure effect on carrot pectinmethylesterase stability and catalytic activity. J. Food Eng. 2007, 78, 755–764. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. Effect of High Hydrostatic Pressure-Temperature Combinations on the Activity of β-Glucanase from Barley Malt. J. Inst. Brew. 2005, 111, 282–289. [Google Scholar] [CrossRef]
- Glucanase. Available online: https://www.wikiwand.com/en/Glucanase (accessed on 27 January 2023).
- Celestino, K.R.S.; Cunha, R.B.; Felix, C.R. Characterization of a β-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry. BMC Biochem. 2006, 7, 23. [Google Scholar] [CrossRef]
- Bhat, M. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ju, L.-K. Sophorolipids-induced cellulase production in cocultures of Hypocrea jecorina Rut C30 and Candida bombicola. Enzym. Microb. Technol. 2009, 44, 107–111. [Google Scholar] [CrossRef]
- Cellulase. Available online: https://en.wikipedia.org/wiki/Cellulase (accessed on 27 January 2023).
- Murao, S.; Nomura, Y.; Yoshikawa, M.; Shin, T.; Oyama, H.; Arai, M. Enhancement of Activities of Cellulases under High Hydrostatic Pressure. Biosci. Biotech. Biochem. 1992, 56, 1366–1367. [Google Scholar] [CrossRef]
- Ribeiro, M.H.L.; Rabaça, M. Cross-Linked Enzyme Aggregates of Naringinase: Novel Biocatalysts for Naringin Hydrolysis. Enzym. Res. 2011, 2011, 851272. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.; Hotchkiss, J. Naringinase Immobilization in Packaging Films for Reducing Naringin Concentration in Grapefruit Juice. J. Food Sci. 1998, 63, 61–65. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.; Calado, A.; Ribeiro, M. High pressure-temperature effects on enzymatic activity: Naringin bioconversion. Food Chem. 2006, 102, 565–570. [Google Scholar] [CrossRef]
- Pedro, H.A.; Alfaia, A.J.; Marques, J.; Vila-Real, H.J.; Calado, A.; Ribeiro, M.H. Design of an immobilized enzyme system for naringin hydrolysis at high-pressure. Enzym. Microb. Technol. 2006, 40, 442–446. [Google Scholar] [CrossRef]
- Marques, J.; Vila-Real, H.; Alfaia, A.; Ribeiro, M. Modelling of the high pressure–temperature effects on naringin hydrolysis based on response surface methodology. Food Chem. 2007, 105, 504–510. [Google Scholar] [CrossRef]
- Chymotrypsin. Available online: https://de.wikipedia.org/wiki/Chymotrypsin_B (accessed on 27 January 2023).
- Jaworek, M.W.; Schuabb, V.; Winter, R. The effects of glycine, TMAO and osmolyte mixtures on the pressure dependent enzymatic activity of α-chymotrypsin. Phys. Chem. Chem. Phys. 2018, 20, 1347–1354. [Google Scholar] [CrossRef]
- Schuabb, V.; Winter, R.; Czeslik, C. Improved activity of α-chymotrypsin on silica particles—A high-pressure stopped-flow study. Biophys. Chem. 2016, 218, 1–6. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Recio, I.; López-Fandiño, R. Influence of high hydrostatic pressure on the proteolysis of β-lactoglobulin A by trypsin. J. Dairy Res. 2006, 73, 121–128. [Google Scholar] [CrossRef]
- Kunugi, S.; Fukuda, M.; Ise, N. Pressure dependence of trypsin-catalyzed hydrolyses of specific substrates. Biochim. Biophys. Acta 1982, 704, 107–113. [Google Scholar] [CrossRef]
- Inouye, K.; Kusano, M.; Hashida, Y.; Minoda, M.; Yasukawa, K. Engineering, expression, purification, and production of recombinant thermolysin. Biotechnol. Annu. Rev. 2007, 13, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Birrane, G.; Bhyravbhatla, B.; Navia, M.A. Synthesis of Aspartame by Thermolysin: An x-Ray Structural Study. ACS Med. Chem. Lett. 2014, 5, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Cholewiak, A.; Adamczyk, K.; Kopyt, M.; Kasztelan, A.; Kwiatkowski, P. High pressure-assisted low-loading asymmetric organocatalytic conjugate addition of nitroalkanes to chalcones. Org. Biomol. Chem. 2018, 16, 4365–4371. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Dudziński, K.; Łyżwa, D. Effect of High Pressure on the Organocatalytic Asymmetric Michael Reaction: Highly Enantioselective Synthesis of γ-Nitroketones with Quaternary Stereogenic Centers. Org. Lett. 2011, 13, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Cholewiak, A.; Kasztelan, A. Efficient and Highly Enantioselective Construction of Trifluoromethylated Quaternary Stereogenic Centers via High-Pressure Mediated Organocatalytic Conjugate Addition of Nitromethane to β,β-Disubstituted Enones. Org. Lett. 2014, 16, 5930–5933. [Google Scholar] [CrossRef]
- Nacsa, E.D.; MacMillan, D.W.C. Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols. J. Am. Chem. Soc. 2018, 140, 3322–3330. [Google Scholar] [CrossRef]
- Łyżwa, D.; Dudziński, K.; Kwiatkowski, P. High-Pressure Accelerated Asymmetric Organocatalytic Friedel–Crafts Alkylation of Indoles with Enones: Application to Quaternary Stereogenic Centers Construction. Org. Lett. 2012, 14, 1540–1543. [Google Scholar] [CrossRef]
- Miyamae, N.; Watanabe, N.; Moritaka, M.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Asymmetric organocatalytic desymmetrization of 4,4-disubstituted cyclohexadienones at high pressure: A new powerful strategy for the synthesis of highly congested chiral cyclohexenones. Org. Biomol. Chem. 2014, 12, 5847–5855. [Google Scholar] [CrossRef]
- Kasztelan, A.; Biedrzycki, M.; Kwiatkowski, P. High-Pressure-Mediated Asymmetric Organocatalytic Hydroxyalkylation of Indoles with Trifluoromethyl Ketones. Adv. Synth. Catal. 2016, 358, 2962–2969. [Google Scholar] [CrossRef]
- Abe, F. Exploration of the Effects of High Hydrostatic Pressure on Microbial Growth, Physiology and Survival: Perspectives from Piezophysiology. Biosci. Biotechnol. Biochem. 2007, 71, 2347–2357. [Google Scholar] [CrossRef]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Gerringer, M.E.; Yancey, P.H.; Tikhonova, O.V.; Vavilov, N.E.; Zgoda, V.G.; Davydov, D.R. Pressure tolerance of deep-sea enzymes can be evolved through increasing volume changes in protein transitions: A study with lactate dehydrogenases from abyssal and hadal fishes. FEBS J. 2020, 287, 5394–5410. [Google Scholar] [CrossRef] [PubMed]
- Siebenaller, J.F.; Somero, G.N. Pressure-adaptive differences in the binding and catalytic properties of muscle-type (M4) lactate dehydrogenases of shallow- and deep-living marine fishes. J. Comp. Physiol. B 1979, 129, 295–300. [Google Scholar] [CrossRef]
- Qin, Q.-L.; Wang, Z.-B.; Su, H.-N.; Chen, X.-L.; Miao, J.; Wang, X.-J.; Li, C.-Y.; Zhang, X.-Y.; Li, P.-Y.; Wang, M.; et al. Oxidation of trimethylamine to trimethylamine N -oxide facilitates high hydrostatic pressure tolerance in a generalist bacterial lineage. Sci. Adv. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Jebbar, M.; Franzetti, B.; Girard, E.; Oger, P. Microbial diversity and adaptation to high hydrostatic pressure in deep-sea hydrothermal vents prokaryotes. Extremophiles 2015, 19, 721–740. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scepankova, H.; Galante, D.; Espinoza-Suaréz, E.; Pinto, C.A.; Estevinho, L.M.; Saraiva, J. High Hydrostatic Pressure in the Modulation of Enzymatic and Organocatalysis and Life under Pressure: A Review. Molecules 2023, 28, 4172. https://doi.org/10.3390/molecules28104172
Scepankova H, Galante D, Espinoza-Suaréz E, Pinto CA, Estevinho LM, Saraiva J. High Hydrostatic Pressure in the Modulation of Enzymatic and Organocatalysis and Life under Pressure: A Review. Molecules. 2023; 28(10):4172. https://doi.org/10.3390/molecules28104172
Chicago/Turabian StyleScepankova, Hana, Diogo Galante, Edelman Espinoza-Suaréz, Carlos A. Pinto, Letícia M. Estevinho, and Jorge Saraiva. 2023. "High Hydrostatic Pressure in the Modulation of Enzymatic and Organocatalysis and Life under Pressure: A Review" Molecules 28, no. 10: 4172. https://doi.org/10.3390/molecules28104172
APA StyleScepankova, H., Galante, D., Espinoza-Suaréz, E., Pinto, C. A., Estevinho, L. M., & Saraiva, J. (2023). High Hydrostatic Pressure in the Modulation of Enzymatic and Organocatalysis and Life under Pressure: A Review. Molecules, 28(10), 4172. https://doi.org/10.3390/molecules28104172