Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K
Abstract
:1. Introduction
2. Results
2.1. Identification of Cathepsin K Inhibitors in the Extract of Chamaecrista Nomame and Analysis of Specificity of Cathepsin K Inhibitors, including Pheophytin a Derivatives
2.1.1. Purification of Cat K Inhibitors from the Methanol Extract of Chamaecrista nomame
2.1.2. Identification of Two Types of Cat K Inhibitor in the Methanol Extract of Chamaecrista nomame and Their Inhibitory Activity towards Cat K and B
2.1.3. Preparation of Pheophytin a Derivatives and Their Inhibitory Activity towards Cat K and Cat B
2.2. Mode of Cathepsin K Inhibition by Pheophytin a and Pheophorbide b and Determination of Kinetic Parameters
2.2.1. Allosteric Inhibition of Cat K by Pheophytin a and Pheophorbide b
2.3. Analysis of Allosteric Binding of Pheophytin a and Pheophorbide b Using AutoDock Vina
2.3.1. Docking Simulation of Pheophytin a and Pheophorbide b to Cat K
2.3.2. Pheophytin a and Pheophorbide b Bind to Each Allosteric Site That Is Different from the Allosteric Sites on Cat K, to Which the Known Allosteric Inhibitors NSC13345 and NSC94914 Bind
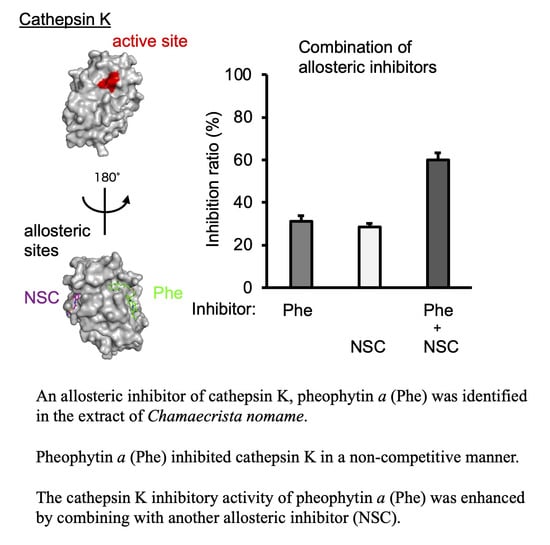
2.4. Additional Inhibition of Cat K by a Combination of Allosteric Inhibitors That Bind to Different Allosteric Sites
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Instruments
4.2. Preparation of Extract of Chamaecrista nomame and Purification of Cat K Inhibitors
4.3. Preparation of Pheophytin a Derivatives from Extract of Spinach
4.4. Measurement of Inhibitory Activity of Inhibitors against Cat K and Cat B
4.5. Kinetic Analysis of Cat K Inhibition
4.6. Docking Study
4.7. Inhibition of Cathepsin K by Combination of Allosteric Inhibitors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Duong, L.T.; Leung, A.T.; Langdahl, B. Cathepsin K Inhibition: A New Mechanism for the Treatment of Osteoporosis. Calcif. Tissue Int. 2016, 98, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Deeks, E.D. Asciminib: First Approval. Drugs 2022, 82, 219–226. [Google Scholar] [CrossRef]
- Hoy, S.M. Deucravacitinib: First Approval. Drugs 2022, 82, 1671–1679. [Google Scholar] [CrossRef]
- Lu, S.; Li, S.; Zhang, J. Harnessing allostery: A novel approach to drug discovery. Med. Res. Rev. 2014, 34, 1242–1285. [Google Scholar] [CrossRef]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef]
- Hatano, T.; Mizuta, S.; Ito, H.; Yoshida, T. C-Glycosidic flavonoids from Cassia occidentalis. Phytochemistry 1999, 52, 1379–1383. [Google Scholar] [CrossRef]
- Hori, H.; Ishitani, O.; Ibuski, T.; Inoue, H. Simple and rapid preparation method of standard samples of pheophytins. Bunseki Kagaku 1994, 43, 939–945. [Google Scholar] [CrossRef]
- Kitaoka, M. Automatic Calculation of the Kinetic Parameters of Enzymatic Reactions with Their Standard Errors Using Microsoft Excel. J. Appl. Glycosci. 2023, 70, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Korenč, M.; Caflisch, A.; Ranganathan, R.; Lenarčič, B.; Baici, A. A novel allosteric mechanism in the cysteine peptidase cathepsin K discovered by computational methods. Nat. Commun. 2014, 5, 3287. [Google Scholar] [CrossRef]
- Novinec, M.; Rebernik, M.; Lenarčič, B. An allosteric site enables fine-tuning of cathepsin K by diverse effectors. FEBS Lett. 2016, 590, 4507–4518. [Google Scholar] [CrossRef]
- Novinec, M.; Lenarčič, B.; Baici, A. Probing the activity modification space of the cysteine peptidase cathepsin K with novel allosteric modifiers. PLoS ONE 2014, 9, e106642. [Google Scholar] [CrossRef]
- Novinec, M. Computational investigation of conformational variability and allostery in cathepsin K and other related peptidases. PLoS ONE 2017, 12, e0182387. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Nishino, K.; Someya, K.; Ksouri, R.; Ishikawa, T.; Isoda, H.; Irie, K.; Nagao, M. Abietane diterpenoids from Salvia officinalis leaves as aryl hydrocarbon receptor ligands. Phytochem. Lett. 2021, 41, 78–82. [Google Scholar] [CrossRef]
- Ternikar, S.G.; Patil, M.B.; Pasha, I.; Khanal, P. Gene set enrichment analysis of α-amylase and α-glucosidase inhibitors of Cassia glauca. J. Diabetes Metab. Disord. 2020, 19, 683–689. [Google Scholar] [CrossRef]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Tsao, H.W.; Chen, Y.Y.; Cheng, S.W.; Hsu, Y.C. A study on the photodynamic properties of chlorophyll derivatives using human hepatocellular carcinoma cells. Photochem. Photobiol. Sci. 2007, 6, 1341–1348. [Google Scholar] [CrossRef]
- Moukheiber, D.; Chitgupi, U.; Carter, K.A.; Luo, D.; Sun, B.; Goel, S.; Ferreira, C.A.; Engle, J.W.; Wang, D.; Geng, J.; et al. Surfactant-Stripped Pheophytin Micelles for Multimodal Tumor Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Aya, T.; Hamilton, A.D. Tetrabiphenylporphyrin-based receptors for protein surfaces show sub-nanomolar affinity and enhance unfolding. Bioorg. Med. Chem. Lett. 2003, 13, 2651–2654. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Hamilton, A.D. Protein surface recognition and proteomimetics: Mimics of protein surface structure and function. Curr. Opin. Chem. Biol. 2005, 9, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Beyett, T.S.; To, C.; Heppner, D.E.; Rana, J.K.; Schmoker, A.M.; Jang, J.; De Clercq, D.J.H.; Gomez, G.; Scott, D.A.; Gray, N.S.; et al. Molecular basis for cooperative binding and synergy of ATP-site and allosteric EGFR inhibitors. Nat. Commun. 2022, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J. The different ways through which specificity works in orthosteric and allosteric drugs. Curr. Pharm. Des. 2012, 18, 1311–1316. [Google Scholar] [CrossRef]
- Feng, L.; Luo, H.; Xu, Z.; Yang, Z.; Du, G.; Zhang, Y.; Yu, L.; Hu, K.; Zhu, W.; Tong, Q.; et al. Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-γ and PPAR-α agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice. Diabetologia 2016, 59, 1276–1286. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Yamamoto, K.; Ito, M.; Nishino, K.; Otsuka, T.; Irie, K.; Nagao, M. Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K. Molecules 2023, 28, 4197. https://doi.org/10.3390/molecules28104197
Sato S, Yamamoto K, Ito M, Nishino K, Otsuka T, Irie K, Nagao M. Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K. Molecules. 2023; 28(10):4197. https://doi.org/10.3390/molecules28104197
Chicago/Turabian StyleSato, Shun, Kana Yamamoto, Moeno Ito, Katsutoshi Nishino, Takanao Otsuka, Kazuhiro Irie, and Masaya Nagao. 2023. "Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K" Molecules 28, no. 10: 4197. https://doi.org/10.3390/molecules28104197
APA StyleSato, S., Yamamoto, K., Ito, M., Nishino, K., Otsuka, T., Irie, K., & Nagao, M. (2023). Enhancement of Inhibitory Activity by Combining Allosteric Inhibitors Putatively Binding to Different Allosteric Sites on Cathepsin K. Molecules, 28(10), 4197. https://doi.org/10.3390/molecules28104197