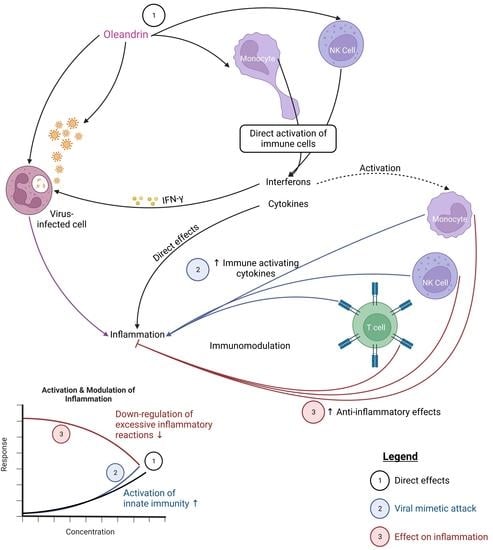
Differential Activities of the Botanical Extract PBI-05204 and Oleandrin on Innate Immune Functions under Viral Challenge Versus Inflammatory Culture Conditions
Abstract
:1. Introduction
2. Results
2.1. Induction of the CD69 and CD25 Activation Markers on Innate Immune Cells
2.2. Enhanced Expression of CD69 and CD25 Activation Markers with Viral Mimetic Challenge
2.3. Direct Induction of Cytokine Production
2.4. Regulation of Cytokines in Context of a Viral Mimetic Challenge
2.5. Regulation of Inflammation
2.6. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Test Products
4.3. Immune Cell Activation
4.4. Production of Cytokines, Chemokines, and Growth Factors
4.5. NK Cell Cytotoxicity towards K562 Human Myelogenous Leukemia Target Cells
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lu, L.; Su, S.; Yang, H.; Jiang, S. Antivirals with common targets against highly pathogenic viruses. Cell 2021, 184, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R. Detecting the emergence of novel, zoonotic viruses pathogenic to humans. Cell Mol. Life Sci. 2015, 72, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, R.A.; Chase, C.C.L.; Matos, J.R.; Abdelsalam, K.; Buterbaugh, R.; Van Holland, S.; Abdelaal, H.; Woolum, A.; Jagannadha Sastry, K. Efficacy of oleandrin and PBI-05204 against bovine viruses of importance to commercial cattle health. Antivir. Chem. Chemother. 2022, 30, 20402066221103960. [Google Scholar] [CrossRef]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- DeWolf, S.; Laracy, J.C.; Perales, M.A.; Kamboj, M.; van den Brink, M.R.M.; Vardhana, S. SARS-CoV-2 in immunocompromised individuals. Immunity 2022, 55, 1779–1798. [Google Scholar] [CrossRef]
- Triggle, C.R.; Bansal, D.; Ding, H.; Islam, M.M.; Farag, E.A.B.A.; Hadi, H.A.; Sultan, A.A. A Comprehensive Review of Viral Characteristics, Transmission, Pathophysiology, Immune Response, and Management of SARS-CoV-2 and COVID-19 as a Basis for Controlling the Pandemic. Front. Immunol. 2021, 12, 631139. [Google Scholar] [CrossRef]
- Hosseini, A.; Hashemi, V.; Shomali, N.; Asghari, F.; Gharibi, T.; Akbari, M.; Gholizadeh, S.; Jafari, A. Innate and adaptive immune responses against coronavirus. Biomed. Pharmacother. 2020, 132, 110859. [Google Scholar] [CrossRef]
- Carty, M.; Guy, C.; Bowie, A.G. Detection of Viral Infections by Innate Immunity. Biochem. Pharmacol. 2021, 183, 114316. [Google Scholar] [CrossRef]
- Hanan, N.; Doud, R.L., Jr.; Park, I.W.; Jones, H.P.; Mathew, S.O. The Many Faces of Innate Immunity in SARS-CoV-2 Infection. Vaccines 2021, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccines Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürst, R.; Zündorf, I.; Dingermann, T. New Knowledge About Old Drugs: The Anti-Inflammatory Properties of Cardiac Glycosides. Planta Med. 2017, 83, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26, 6197. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Yousef, S.M.; Abu-Yousef, I.A.; Nasrallah, G.K. Immunomodulatory and Anti-Inflammatory Effects of Berberine in Lung Tissue and its Potential Application in Prophylaxis and Treatment of COVID-19. Front Biosci. (Landmark Ed) 2022, 27, 166. [Google Scholar] [CrossRef]
- Newman, R.A.; Sastry, K.J.; Arav-Boger, R.; Cai, H.; Matos, R.; Harrod, R. Antiviral Effects of Oleandrin. J. Exp. Pharmacol. 2020, 12, 503–515. [Google Scholar] [CrossRef]
- Talia, D.M.; Deliyanti, D.; Agrotis, A.; Wilkinson-Berka, J.L. Inhibition of the Nuclear Receptor RORγ and Interleukin-17A Suppresses Neovascular Retinopathy: Involvement of Immunocompetent Microglia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Karaś, K.; Sałkowska, A.; Walczak-Drzewiecka, A.; Ryba, K.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. The cardenolides strophanthidin, digoxigenin and dihydroouabain act as activators of the human RORγ/RORγT receptors. Toxicol. Lett. 2018, 295, 314–324. [Google Scholar] [CrossRef]
- Manna, S.K.; Sah, N.K.; Newman, R.A.; Cisneros, A.; Aggarwal, B.B. Oleandrin suppresses activation of nuclear transcription factor-kappaB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000, 60, 3838–3847. [Google Scholar] [PubMed]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.D.; Ning, J.; Sun, S.L. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. 2021, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, M.; Mao, Y.; Zhang, L.; Wang, X.; Li, S.; Qin, L.; Xu, J.; Hu, L.; Qiao, H. Localized Microsphere/Hydrogel for Tumor Immunotherapy of Cardiac Glycoside with Minimal Toxicity. ACS Appl. Mater. Interfaces 2023, 15, 578–590. [Google Scholar] [CrossRef]
- Benson, K.F.; Newman, R.A.; Jensen, G.S. Antioxidant, anti-inflammatory, anti-apoptotic, and skin regenerative properties of an Aloe vera-based extract of Nerium oleander leaves (nae-8(®)). Clin. Cosmet. Investig. Dermatol. 2015, 8, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Benson, K.F.; Newman, R.A.; Jensen, G.S. Water-soluble egg membrane enhances the immune activating properties of an Aloe vera-based extract of Nerium oleander leaves. Clin. Cosmet. Investig. Dermatol. 2016, 9, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Phillips, F.; Jensen, G.S.; Showman, L.; Tonda, R.; Horst, G.; Levine, R. Particulate and solubilized β-glucan and non-βglucan fractions of Euglena gracilis induce pro- and anti-inflammatory innate immune cell responses and exhibit antioxidant properties. J. Inflamm Res. 2019, 12, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The mycelium of the Trametes versicolor (Turkey Tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro. BMC Complement. Altern. Med. 2019, 19, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.; Taylor, A.; Nally, R.; Benson, K.F.; Stamets, P.; Jensen, G.S. Differential Immune Activating, Anti-Inflammatory, and Regenerative Properties of the Aqueous, Ethanol, and Solid Fractions of a Medicinal Mushroom Blend. J. Inflamm. Res. 2020, 13, 117–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Ko, Y.S.; Rugira, T.; Jin, H.; Park, S.W.; Kim, H.J. Oleandrin and Its Derivative Odoroside A, Both Cardiac Glycosides, Exhibit Anticancer Effects by Inhibiting Invasion via Suppressing the STAT-3 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3350. [Google Scholar] [CrossRef] [Green Version]
- Eroğlu Güneş, C.; Seçer Çelik, F.; Seçme, M.; Elmas, L.; Dodurga, Y.; Kurar, E. Glycoside oleandrin downregulates toll-like receptor pathway genes and associated miRNAs in human melanoma cells. Gene 2022, 843, 146805. [Google Scholar] [CrossRef]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Yarchoan, R.; Redfield, R.; Cleghorn, F.; Blattner, W.A.; Garzino-Demo, A.; Colombini-Hatch, S.; Margolis, D.; Gallo, R.C. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proc. Natl. Acad. Sci. USA 2000, 97, 13812–13817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitzer, C.; Krüger, C.; Plaas, C.; Kirsch, F.; Dittgen, T.; Müller, R.; Laage, R.; Kastner, S.; Suess, S.; Spoelgen, R.; et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain 2008, 131 Pt 12, 3335–3347. [Google Scholar] [CrossRef] [PubMed]
- Quiding-Järbrink, M.; Nordström, I.; Granström, G.; Kilander, A.; Jertborn, M.; Butcher, E.C.; Lazarovits, A.I.; Holmgren, J.; Czerkinsky, C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Investig. 1997, 99, 1281–1286. [Google Scholar] [CrossRef]
- Quiding-Järbrink, M.; Ahlstedt, I.; Lindholm, C.; Johansson, E.L.; Lönroth, H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut 2001, 49, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Boyaka, P.N.; McGhee, J.R.; Czerkinsky, C.; Mestecky, J. Mucosal Vaccines: An Overview. Mucosal Immunol. 2005, 1, 855–874. [Google Scholar] [CrossRef]
- Plante, K.S.; Dwivedi, V.; Plante, J.A.; Fernandez, D.; Mirchandani, D.; Bopp, N.; Aguilar, P.V.; Park, J.G.; Tamayo, P.P.; Delgado, J.; et al. Antiviral activity of oleandrin and a defined extract of Nerium oleander against SARS-CoV-2. Biomed. Pharmacother. 2021, 138, 111457. [Google Scholar] [CrossRef]
- Boff, L.; Schneider, N.F.Z.; Munkert, J.; Ottoni, F.M.; Ramos, G.S.; Kreis, W.; Braga, F.C.; Alves, R.J.; de Pádua, R.M.; Simões, C.M.O. Elucidation of the mechanism of anti-herpes action of two novel semisynthetic cardenolide derivatives. Arch Virol. 2020, 165, 1385–1396. [Google Scholar] [CrossRef]
- Kapoor, A.; Cai, H.; Forman, M.; He, R.; Shamay, M.; Arav-Boger, R. Human cytomegalovirus inhibition by cardiac glycosides: Evidence for involvement of the HERG gene. Antimicrob. Agents Chemother. 2012, 56, 4891–4899. [Google Scholar] [CrossRef] [Green Version]
- Raviprakash, N.; Manna, S.K. Short-term exposure to oleandrin enhances responses to IL-8 by increasing cell surface IL-8 receptors. Br. J. Pharmacol. 2014, 171, 3339–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, D.E.; He, D.N.; Yang, P.; Johansen, M.; Newman, R.A.; Lo, D.C. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J. Neurochem. 2011, 119, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Yang, P.; Rossetti, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Conway, T.L.; Chakraborty, S.; Marampon, F.; Mattei, V.; et al. The Botanical Drug PBI-05204, a Supercritical CO2 Extract of Nerium Oleander, Inhibits Growth of Human Glioblastoma, Reduces Akt/mTOR Activities, and Modulates GSC Cell-Renewal Properties. Front Pharmacol. 2020, 11, 552428. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, G.S.; Yu, L.; Iloba, I.; Cruickshank, D.; Matos, J.R.; Newman, R.A. Differential Activities of the Botanical Extract PBI-05204 and Oleandrin on Innate Immune Functions under Viral Challenge Versus Inflammatory Culture Conditions. Molecules 2023, 28, 4799. https://doi.org/10.3390/molecules28124799
Jensen GS, Yu L, Iloba I, Cruickshank D, Matos JR, Newman RA. Differential Activities of the Botanical Extract PBI-05204 and Oleandrin on Innate Immune Functions under Viral Challenge Versus Inflammatory Culture Conditions. Molecules. 2023; 28(12):4799. https://doi.org/10.3390/molecules28124799
Chicago/Turabian StyleJensen, Gitte S., Liu Yu, Ifeanyi Iloba, Dina Cruickshank, Jose R. Matos, and Robert A. Newman. 2023. "Differential Activities of the Botanical Extract PBI-05204 and Oleandrin on Innate Immune Functions under Viral Challenge Versus Inflammatory Culture Conditions" Molecules 28, no. 12: 4799. https://doi.org/10.3390/molecules28124799
APA StyleJensen, G. S., Yu, L., Iloba, I., Cruickshank, D., Matos, J. R., & Newman, R. A. (2023). Differential Activities of the Botanical Extract PBI-05204 and Oleandrin on Innate Immune Functions under Viral Challenge Versus Inflammatory Culture Conditions. Molecules, 28(12), 4799. https://doi.org/10.3390/molecules28124799