Conversion of Methanol to Para-Xylene over ZSM-5 Zeolites Modified by Zinc and Phosphorus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of P/ZSM-5 Systems from Different Phosphorus Sources
2.1.1. Synthesis and Characterization of P/ZSM-5 Systems
| Samples | Si/Al | B Acids (mmol·g−1) | L Acids (mmol·g−1) | L/B | SB (m2·g−1) | Se (m2·g−1) | Vt (cm3·g−1) | Vmic (cm3·g−1) | Microporosity (%) |
|---|---|---|---|---|---|---|---|---|---|
| HZSM-5 | 36 | 0.170 | 0.054 | 0.32 | 381 | 13 | 0.21 | 0.18 | 85.7 |
| 3P-H3PO4 | 36 | 0.073 | 0.009 | 0.12 | 285 | 8 | 0.16 | 0.13 | 81.2 |
| 3P-(NH4)2HPO4 | 36 | 0.067 | 0.008 | 0.12 | 290 | 8 | 0.17 | 0.15 | 88.2 |
| 3P-(NH4)3PO4 | 37 | 0.036 | 0.005 | 0.14 | 225 | 9 | 0.13 | 0.11 | 84.6 |
2.1.2. Catalytic Performance of P/ZSM-5 in MTA
2.2. Design of Zn-P/ZSM-5 Systems
2.2.1. Synthesis and Characterization of Zn-P/ZSM-5 Systems
2.2.2. Catalytic Performance of Zn-P/ZSM-5 in MTA
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Y.Y.; Li, Y.F.; Chen, L.; Au, C.T.; Yin, S.F. A new catalytic process for the synthesis of para-xylene through benzene methylation with CH3Br. Catal. Commun. 2014, 54, 6–10. [Google Scholar] [CrossRef]
- Yang, D.H.; Wang, X.B.; Shi, B.B.; Wu, Z.H.; Li, X.F.; Dou, T. Synthesis of ZSM-5/EU-1 Composite Zeolite and Its Application in Conversion of Methanol to Xylene. J. Inorg. Mater. 2014, 29, 357–363. [Google Scholar]
- Wang, Y.; Chang, Y.; Liu, M.; Zhang, A.; Guo, X. A Facile Strategy to Prepare Shaped ZSM-5 Catalysts with Enhanced Para-Xylene Selectivity and Stability for Toluene Methylation: The Effect of In Situ Modification by Attapulgite. Molecules 2019, 24, 3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.G.; Qiu, M.H.; Hu, S.W.; Chen, X.Q.; Zeng, G.F.; Liu, Z.Y.; Sun, Y.H. Stable and efficient aromatic yield from methanol over alkali treated hierarchical Zn-containing HZSM-5 zeolites. Microporous Mesoporous Mater. 2016, 231, 110–116. [Google Scholar] [CrossRef]
- Niu, X.J.; Bai, Y.; Du, Y.E.; Qi, H.X.; Chen, Y.Q. Size controllable synthesis of ZSM-5 zeolite and its catalytic performance in the reaction of methanol conversion to aromatics. R. Soc. Open Sci. 2022, 9, 211284. [Google Scholar] [CrossRef]
- Bjørgen, M.; Akyalcin, S.; Olsbye, U.; Benard, S.; Kolboe, S.; Svelle, S. Methanol to hydrocarbons over large cavity zeolites: Toward a unified description of catalyst deactivation and the reaction mechanism. J. Catal. 2010, 275, 170–180. [Google Scholar] [CrossRef]
- Li, N.; Meng, C.; Liu, D. Deactivation kinetics with activity coefficient of the methanol to aromatics process over modified ZSM-5. Fuel 2018, 233, 283–290. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, A.; Wu, X.; Hai, G.; Hu, J.; Li, T.; Li, G. The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol. Microporous Mesoporous Mater. 2011, 143, 435–442. [Google Scholar] [CrossRef]
- Niu, X.J.; Gao, J.; Wang, K.; Miao, Q.; Dong, M.; Wang, G.F.; Fan, W.B.; Qin, Z.F.; Wang, J.G. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics. Fuel Process Technol. 2017, 157, 99–107. [Google Scholar] [CrossRef]
- Fu, T.J.; Shao, J.; Li, Z. Catalytic synergy between the low Si/Al ratio Zn/ZSM-5 and high Si/Al ratio HZSM-5 for high-performance methanol conversion to aromatics. Appl. Catal. B 2021, 291, 120098. [Google Scholar] [CrossRef]
- Wang, K.; Dong, M.; Niu, X.; Li, J.; Qin, Z.; Fan, W.; Wang, J. Highly active and stable Zn/ZSM-5 zeolite catalyst for the conversion of methanol to aromatics: E_ect of support morphology. Catal. Sci. Technol. 2018, 8, 5646–5656. [Google Scholar] [CrossRef]
- Lai, P.C.; Chen, C.H.; Hsu, H.Y.; Lee, C.H.; Lin, Y.C. Methanol aromatization over Ga-doped desilicated HZSM-5. RSC Adv. 2016, 6, 67361–67371. [Google Scholar] [CrossRef]
- Freeman, D.; Wells, R.P.K.; Hutchings, G.J. Conversion of Methanol to Hydrocarbons over Ga2O3/H-ZSM-5 and Ga2O3/WO3 Catalysts. J. Catal. 2002, 205, 358–365. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chen, Y.Y.; Lin, Y.C. Ga-Substituted Nanoscale HZSM-5 in Methanol Aromatization: The Cooperative Action of the Brønsted Acid and the Extra-Framework Ga Species. Ind. Eng. Chem. Res. 2018, 57, 7742–7751. [Google Scholar] [CrossRef]
- Liu, C.; Uslamin, E.A.; Khramenkova, E.; Sireci, E.; Ouwehand, L.T.L.J.; Ganapathy, S.; Kapteijn, F.; Pidko, E.A. High Stability of Methanol to Aromatic Conversion over Bimetallic Ca,Ga-Modified ZSM-5. ACS Catal. 2022, 12, 3189–3200. [Google Scholar] [CrossRef]
- Inoue, Y.; Nakashiro, K.; Ono, Y. Selective conversion of methanol into aromatic hydrocarbons over silver-exchanged ZSM-5 zeolites. Microporous Mater. 1995, 4, 379–383. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Ji, K.; Xun, J.; Liu, P.; Song, Q.; Gao, J.; Zhang, K.; Li, J. The study of methanol aromatization on transition metal modified ZSM-5 catalyst. Chin. J. Chem. Eng. 2018, 26, 1949–1953. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liu, Y.; Li, J.W. In Situ Synthesis of Metal-Containing ZSM-5 and Its Catalytic Performance in Aromatization of Methanol. ACS Omega 2022, 7, 24241–24248. [Google Scholar] [CrossRef]
- Zhou, F.; Ma, H.X.; Han, Z.; Ma, F.W.; Wu, G. Synthesis of hierarchical GaZSM-5 zeolites by the HCl treatment method and their catalytic performance in methanol aromatization. New J. Chem. 2021, 45, 17108–17115. [Google Scholar] [CrossRef]
- Teng, H.; Wang, J.; Ren, X.Q.; Chen, D.M. Disproportionation of Toluene by Modified ZSM-5 Zeolite Catalysts with High Shape-selectivity Prepared Using Chemical Liquid Deposition with Tetraethyl Orthosilicate. Chin. J. Chem. Eng. 2011, 19, 292–298. [Google Scholar] [CrossRef]
- Janardhan, H.L.; Shanbhag, G.V.; Halgeri, A.B. Shape-selective catalysis by phosphate modified ZSM-5: Generation of new acid sites with pore narrowing. Appl. Catal. A 2014, 471, 12–18. [Google Scholar] [CrossRef]
- Das, J.; Bhat, Y.S.; Halgeri, A.B. Selective Toluene Disproportionation over Pore Size Controlled MFI Zeolite. Ind. Eng. Chem. Res. 1994, 33, 246–250. [Google Scholar] [CrossRef]
- Lu, P.; Fei, Z.; Li, L.; Feng, X.; Ji, W.; Ding, W.; Chen, Y.; Yang, W.; Xie, Z. Effects of controlled SiO2 deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol. Appl. Catal. A 2013, 453, 302–309. [Google Scholar] [CrossRef]
- Li, J.H.; Tong, K.; Xi, Z.W.; Yuan, Y.; Hu, Z.H.; Zhu, Z.R. Highly-efficient conversion of methanol to p-xylene over shape-selective Mg–Zn–Si-HZSM-5 catalyst with fine modification of pore-opening and acidic properties. Catal. Sci. Technol. 2016, 6, 4802–4813. [Google Scholar] [CrossRef]
- Zhang, J.G.; Qian, W.Z.; Kong, C.Y.; Wei, F. Increasing para-Xylene Selectivity in Making Aromatics from Methanol with a Surface-Modified Zn/P/ZSM-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Li, H.; Li, X.G.; Xiao, W.D. Collaborative Effect of Zinc and Phosphorus on the Modified HZSM-5 Zeolites in the Conversion of Methanol to Aromatics. Catal. Lett. 2021, 151, 955–965. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhu, X.L.; Zhang, S.H.; Cheng, M.; Yu, M.X.; Wang, G.W.; Li, C.Y. Selective production of para-xylene and light olefins from methanol over the mesostructured Zn–Mg–P/ZSM-5 catalyst. Catal. Sci. Technol. 2019, 9, 316–326. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zhang, J.Y.; Cheng, M.; Wang, G.W.; Yu, M.X.; Li, C.Y. Methanol Aromatization over Mg−P-Modified [Zn,Al]ZSM-5 Zeolites for Efficient Coproduction of para-Xylene and Light Olefins. Ind. Eng. Chem. Res. 2019, 58, 19446–19455. [Google Scholar] [CrossRef]
- Jia, Y.M.; Wang, J.W.; Zhang, K.; Ding, C.M. Highly shape-selective Zn-P/HZSM-5 zeolite catalyst for methanol conversion to light aromatics. Appl Organomet Chem. 2020, 34, 5932. [Google Scholar] [CrossRef]
- Pan, D.; Song, X.; Yang, X.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. Efficient and selective conversion of methanol to para-xylene over stable H[Zn,Al]ZSM-5/SiO2 composite catalyst. Appl. Catal. A 2018, 557, 15–24. [Google Scholar] [CrossRef]
- Miyake, K.; Hirota, Y.; Ono, K.; Uchida, Y.; Tanaka, S.; Nishiyama, N. Direct and selective conversion of methanol to para-xylene over Zn ion doped ZSM-5/silicalite-1 core-shell zeolite catalyst. J. Catal. 2016, 342, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.J.; Wang, K.; Bai, Y.; Du, Y.E.; Chen, Y.Q.; Dong, M.; Fan, W.B. Selective Formation of Para-Xylene by Methanol Aromatization over Phosphorous Modified ZSM-5 Zeolites. Catalysts 2020, 10, 484. [Google Scholar] [CrossRef]
- Su, X.; Wang, G.; Bai, X.; Wu, W.; Xiao, L.; Fang, Y.; Zhang, J. Synthesis of nanosized HZSM-5 zeolites isomorphously substituted by gallium and their catalytic performance in the aromatization. Chem. Eng. J. 2016, 293, 365–375. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Liu, Z.; Peng, W.; Liu, Y.; Liu, C. Correlation between H-ZSM-5 crystal size and catalytic performance in the methanol-to-aromatics reaction. Chin. J. Catal. 2017, 38, 683–690. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Martínez-Triguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Xie, Z.; Jin, W.; Yang, W.; Chen, Q.; Tang, Y. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene. J. Catal. 2007, 248, 29–37. [Google Scholar] [CrossRef]
- Madeira, F.F.; Tayeb, K.B.; Pinard, L.; Vezin, H.; Maury, S.; Cadran, N. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal. A 2012, 443–444, 171–180. [Google Scholar] [CrossRef]
- ZVédrine, J.C.; Auroux, A.; Dejaifve, P.; Ducarme, V.; Hoser, H.; Zhou, S. Catalytic and physical properties of phosphorus-modified ZSM-5 zeolite. J. Catal. 1982, 73, 147–160. [Google Scholar] [CrossRef]
- Caeiro, G.; Magnoux, P.; Lopes, J.M.; Ribeiro, F.R.; Menezes, S.M.C.; Costa, A.F.; Cerqueira, H.S. Stabilization effect of phosphorus on steamed H-MFI zeolites. Appl. Catal. A 2006, 314, 160–171. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Bao, X. Conversion of Methanol to Hydrocarbons over Phosphorus-modified ZSM-5/ZSM-11 Intergrowth Zeolites. Catal. Lett. 2010, 134, 124–130. [Google Scholar] [CrossRef]
- Ghiaci, M.; Abbaspur, A.; Arshadi, M.; Aghabarari, B. Internal versus external surface active sites in ZSM-5 zeolite: Part 2: Toluene alkylation with methanol and 2-propanol catalyzed by modified and unmodified H3PO4/ZSM-5. Appl. Catal. A 2007, 316, 32–46. [Google Scholar] [CrossRef]
- Takahashi, A.; Xia, W.; Nakamura, I.; Shimada, H.; Fujitani, T. Effects of added phosphorus on conversion of ethanol to propylene over ZSM-5 catalysts. Appl. Catal. A 2012, 423–424, 162–167. [Google Scholar] [CrossRef]
- Kim, J.; Cho, K.; Lee, S.; Ryoo, R. Mesopore wall-catalyzed Friedel–Crafts acylation of bulky aromatic compounds in MFI zeolite nanosponge. Catal. Today 2015, 243, 103–108. [Google Scholar] [CrossRef]
- Bibby, D.M.; Milestone, N.B.; Patterson, J.E.; Aldridge, L.P. Coke formation in zeolite ZSM-5. J. Catal. 1986, 97, 493–502. [Google Scholar] [CrossRef]
- Kim, J.; Choi, M.; Ryoo, R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process. J. Catal. 2010, 269, 219–228. [Google Scholar] [CrossRef]
- Xue, N.; Chen, X.; Nie, L.; Guo, X.; Ding, W.; Chen, Y.; Gu, M.; Xie, Z. Understanding the enhancement of catalytic performance for olefin cracking: Hydrothermally stable acids in P/HZSM-5. J. Catal. 2007, 248, 20–28. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Berndt, H.; Lietz, G.; Völter, J. Zinc promoted H-ZSM-5 catalysts for conversion of propane to aromatics II. Nature of the active sites and their activation. Appl. Catal. A 1996, 146, 365–379. [Google Scholar] [CrossRef]
- Berndt, H.; Lietz, G.; Lücke, B.; Völter, J. Zinc promoted H-ZSM-5 catalysts for conversion of propane to aromatics I. Acidity and activity. Appl. Catal. A 1996, 146, 351–363. [Google Scholar] [CrossRef]
- Ilias, S.; Khare, R.; Malek, A.; Bhan, A. A descriptor for the relative propagation of the aromatic- and olefin-based cycles in methanol-to-hydrocarbons conversion on H-ZSM-5. J. Catal. 2013, 303, 135–140. [Google Scholar] [CrossRef]
- Svelle, S.; Joensen, F.; Nerlov, J.; Olsbye, U.; Lillerud, K.-P.; Kolboe, S.; Bjørgen, M. Conversion of Methanol into Hydrocarbons over Zeolite H-ZSM-5: Ethene Formation Is Mechanistically Separated from the Formation of Higher Alkenes. J. Am. Chem. Soc. 2006, 128, 14770–14771. [Google Scholar] [CrossRef] [PubMed]
- Ghiaci, M.; Abbaspur, A.; Kalbasi, R.J. Internal versus external surface active sites in ZSM-5 zeolite Part 1. Fries rearrangement catalyzed by modified and unmodified H3PO4/ZSM-5. Appl. Catal. A 2006, 298, 32–39. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Dai, C.; Du, N.; Li, T.; Wang, R.; Peng, P.; Sun, H. Zn-P Co-Modified Hierarchical ZSM-5 Zeolites Directly Synthesized via Dry Gel Conversion for Enhanced Methanol to Aromatics Reaction. Catalysts 2021, 11, 1388. [Google Scholar] [CrossRef]









| Catalysts | Modification Method | Modifiers | Reaction Conditions | Modification Effect | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | WHSV a (h−1) | Lifetime b (h) | SArom c (%) | PX/X (%) | PX Yield (%) | ||||
| H[Zn, Al]ZSM-5 | Direct synthesis | Zn(NO3)2, ammonia | 437 | 3.2 | 32/37 | 31.0/46.7 (BTX) | – | – | [8] |
| Zn/HZSM-5 | Impregnation | Zn(NO3)2 | 390 | 3.2 | 170/80 | 36.2/45.8 | 23.8 | – | [9] |
| Ga/HZSM-5 | Alkaline treatment, impregnation | Ga(NO3)3 | 475 | 1.06 | 11.5/9.0 | 38.7/51.7 | 17.5 | – | [12] |
| Zn/ZSM-5 | Direct synthesis | ZnCl2 | 450 | 5 | >5 | 41/56 | – | – | [19] |
| Ga-ZSM-5 | Direct synthesis, HCl treatment | Ga2O3 | 400 | 10 | 18/30 | 18.2/29.3 (C6, C6+) | – | – | [20] |
| Mg/Zn/Si/HZSM-5 | Si-CLD d, Zn, Mg-impregnation | PPMS e, Zn(NO3)2, Mg(NO3)2 | 460 | 1.0 | >12 | 38.4/57.3 | 98.9 | 21.24 | [25] |
| Zn/P/Si/ZSM-5 | Zn,P-impregnated, Si-CLD | Zn(NO3)2, H3PO4, TEOS | 475 | 0.79 | – | 44.6/61.7 | 89.6 | – | [26] |
| Zn-P/HZSM-5 | Impregnation | Zn3(PO4)2, H3PO4 | 480 | 4.7 | 13 | 25/61.2 | – | – | [27] |
| Zn-Mg-P/ZSM-5 | Alkaline treatment, impregnation | Zn(NO3)2, Mg(NO3)2, H3PO4 | 400 | 2.4 | 50 | 35.9/43.6 | 90.8 | 19 | [28] |
| Zn-P/HZSM-5 | P-impregnation, Zn-ion exchange | H3PO4, ZnSiF6 | 400 | 0.7 | 462 f | 35.6/46.8 (BTX) | – | – | [30] |
| H[Zn,Al]ZSM-5/SiO2 | Zn-direct synthesis, Si-CLD | Zn(NO3)2, TEOS | 425 | 2.5 | 34/24 | 33.8/40.0 | 95.6 | 18.2 | [31] |
| Zn/ZSM-5/silicalite-1 | Zn-ion exchanged, Si-direct synthesis | Zn(NO3)2, fumed silica | 400 | 0.74 | – | – | 99 | 40.7 | [32] |
| Catalysts | Conv.MeOH a | Production-Selectivity (%) | Xylene in Aromatics | PX in X | Yield of PX c | ||||
|---|---|---|---|---|---|---|---|---|---|
| C1−~C4− | C2=~C5= | C5+ Non-Aromatics | Aromatics | Others b | - | - | - | ||
| HZSM-5 | 99.8 | 37.9 | 8.2 | 19.1 | 33.3 | 1.5 | 43.1 | 23.8 | 3.4 |
| 1Zn | 99.5 | 28.1 | 14.9 | 18.6 | 36.7 | 1.7 | 43.0 | 24.4 | 3.8 |
| 1P-1Zn | 98.8 | 18.9 | 32.0 | 20.1 | 28.2 | 0.8 | 45.7 | 40.1 | 5.1 |
| 3P-1Zn | 83.8 | 14.0 | 46.4 | 19.9 | 18.7 | 1 | 55.6 | 78.2 | 6.8 |
| 5P-1Zn | 77.8 | 11.7 | 57.1 | 14.5 | 15.9 | 0.8 | 60.7 | 90.1 | 6.8 |
| 8P-1Zn | 51.2 | 9.7 | 71.6 | 9.2 | 9.2 | 0.3 | 52.4 | 91.3 | 2.3 |
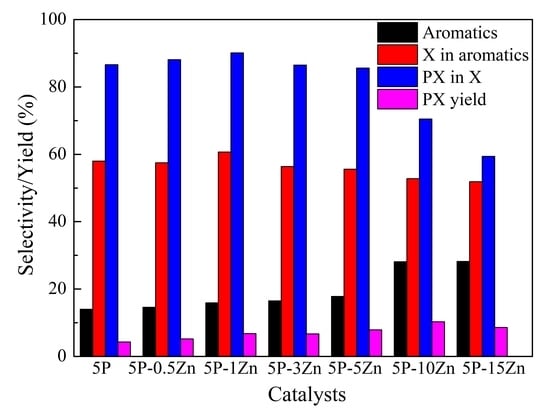
| 5P | 61.7 | 12.7 | 59.6 | 13.1 | 14.0 | 0.6 | 58.0 | 86.6 | 4.3 |
| 5P-0.5Zn | 69.9 | 12.4 | 56.1 | 15.6 | 14.6 | 1.3 | 57.5 | 88.1 | 5.2 |
| 5P-1Zn | 77.8 | 11.7 | 57.1 | 14.5 | 15.9 | 0.8 | 60.7 | 90.1 | 6.8 |
| 5P-3Zn | 83.4 | 13.8 | 52.2 | 16.7 | 16.5 | 0.8 | 56.4 | 86.5 | 6.7 |
| 5P-5Zn | 92.7 | 13.1 | 46.2 | 22.0 | 17.8 | 0.9 | 55.6 | 85.6 | 7.9 |
| 5P-8Zn | 96.6 | 28.5 | 18.7 | 28.8 | 23.0 | 1 | 51.4 | 76.8 | 8.8 |
| 5P-10Zn | 98.4 | 18.8 | 21.8 | 30.5 | 28.1 | 0.8 | 52.8 | 70.5 | 10.3 |
| 5P-15Zn | 99.1 | 17.0 | 28.3 | 25.2 | 28.2 | 1.3 | 51.9 | 59.4 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Niu, X.; Du, Y.-E.; Chen, Y. Conversion of Methanol to Para-Xylene over ZSM-5 Zeolites Modified by Zinc and Phosphorus. Molecules 2023, 28, 4890. https://doi.org/10.3390/molecules28134890
Bai Y, Niu X, Du Y-E, Chen Y. Conversion of Methanol to Para-Xylene over ZSM-5 Zeolites Modified by Zinc and Phosphorus. Molecules. 2023; 28(13):4890. https://doi.org/10.3390/molecules28134890
Chicago/Turabian StyleBai, Yang, Xianjun Niu, Yi-En Du, and Yongqiang Chen. 2023. "Conversion of Methanol to Para-Xylene over ZSM-5 Zeolites Modified by Zinc and Phosphorus" Molecules 28, no. 13: 4890. https://doi.org/10.3390/molecules28134890
APA StyleBai, Y., Niu, X., Du, Y. -E., & Chen, Y. (2023). Conversion of Methanol to Para-Xylene over ZSM-5 Zeolites Modified by Zinc and Phosphorus. Molecules, 28(13), 4890. https://doi.org/10.3390/molecules28134890