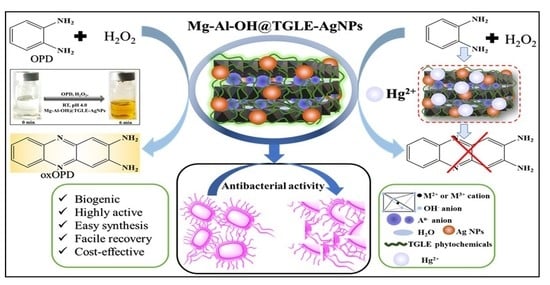
Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
2.2. Spectroscopic and Microscopic Analysis of Mg-Al-OH@TGEL-AgNPs Nanocatalyst
2.2.1. GC-MS and Qualitative Analysis of TGLE
2.2.2. UV-Visible Analysis of TGLE
2.2.3. FT-IR Spectroscopy
2.2.4. FE-SEM Analysis
2.2.5. EDS Analysis
2.2.6. p-XRD Analysis
2.2.7. Thermogravimetric Analysis
2.3. Peroxidase-like Activity of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
2.3.1. Effect of Parameters in the Peroxidase-like Activity of the Mg-Al-OH@TGLE-AgNPs Nanocatalyst
2.3.2. Kinetic Analysis of the Peroxidase-like Activity of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
2.4. Application of Mg-Al-OH@TGLE-AgNPs Nanocatalyst in Sensing of Mercury
2.5. Antibacterial Activity of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
3. Experimental Section
3.1. Materials
3.2. Instrumentation and Analyses
3.3. Synthesis of Mg-Al Layered Double Hydroxides (Mg-Al-OH) (1)
3.4. Preparation of Tectona Grandis Leaves Extract (TGLE) (2)
3.5. Green Synthesis of Ag NPs Entrenched TGLE Dispersed on Mg-Al-OH (Mg-Al-OH@TGLE-AgNPs) (3)
3.6. Peroxidase-like Activity of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
3.7. Mercury Detection Using Mg-Al-OH@TGLE-AgNPs Nanocatalyst
3.8. Anti-Bacterial Activity of Mg-Al-OH@TGLE-AgNPs Nanocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Tandon, P.K. Catalysis: A brief review on nano-catalyst. J. Energy Chem. 2014, 2, 106–115. [Google Scholar]
- Balasurya, S.; Syed, A.; Thomas, A.M.; Marraiki, N.; Elgorban, A.M.; Raju, L.L.; Das, A.; Khan, S.S. Rapid colorimetric detection of mercury using silver nanoparticles in the presence of methionine. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117712. [Google Scholar] [CrossRef] [PubMed]
- Sampatkumar, H.G.; Antony, A.M.; Trivedi, M.; Sharma, M.; Ghate, M.; Baidya, M.; Dateer, R.B.; Patil, S.A. In situ biosynthesis of palladium nanoparticles on banana leaves extract-coated graphitic carbon nitride: An efficient and reusable heterogeneous catalyst for organic transformations and antimicrobial agent. Biomass Convers. Biorefin. 2022, 1–22. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mahmoudi-Gom Yek, S.; Motahharifar, N.; Ghafori Gorab, M. Recent developments in the plant-mediated green synthesis of Ag-based nanoparticles for environmental and catalytic applications. Chem. Rec. 2019, 19, 2436–2479. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A. Recent advances in synthesis and degradation of lignin and lignin nanoparticles and their emerging applications in nanotechnology. Materials 2022, 15, 953. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Jiang, Y.; Chi, M.; Nie, G.; Lu, X.; Wang, C. Palladium nanoparticles modified electrospun CoFe2O4 nanotubes with enhanced peroxidase-like activity for colorimetric detection of hydrogen peroxide. RSC Adv. 2016, 6, 33636–33642. [Google Scholar] [CrossRef]
- Antony, A.M.; Kandathil, V.; Kempasiddaiah, M.; Dateer, R.B.; Patil, S.A. Magnetic nanoparticles embedded hexagonal boron nitride tethered N-heterocyclic carbene-palladium(II): An efficient and reusable magnetic catalyst for fluoride-free Hiyama cross-coupling and 4-nitrophenol reduction reactions. J. Phys. Chem. Solids 2023, 177, 111283. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Nanoporous silica-supported organocatalyst: A heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 2014, 4, 28238–28248. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zeng, G.; Huang, L.; Huang, Q.; Liu, M.; Xu, D.; Huang, H.; Yang, Z.; Deng, F.; Zhang, X.; Wei, Y. Rapid synthesis of MoS2-PDA-Ag nanocomposites as heterogeneous catalysts and antimicrobial agents via microwave irradiation. Appl. Surf. Sci. 2018, 459, 588–595. [Google Scholar] [CrossRef]
- Nagarajan, D.; Venkatanarasimhan, S. Copper (II) oxide nanoparticles coated cellulose sponge—An effective heterogeneous catalyst for the reduction of toxic organic dyes. Environ. Sci. Pollut. Res. 2019, 26, 22958–22970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z. Synthesis and characterisation of nanohybrids of olsalazine-intercalated Al–Mg layered double hydroxide. J. Exp. Nanosci. 2012, 7, 412–425. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.J.; Choi, A.-J.; Kim, T.-H.; Kim, T.-i.; Oh, J.-M. Layered double hydroxide nanomaterials encapsulating Angelica gigas Nakai extract for potential anticancer nanomedicine. Front. Pharmacol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Mullaj, K.; Price, A.; Wei, K.; Luo, Q.; Thanneeru, S.; Sun, S.; He, J. Polymer N-Heterocyclic Carbene (NHC) Ligands for Silver Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 55227–55237. [Google Scholar] [CrossRef]
- Garg, A.; Khupse, N.; Bordoloi, A.; Sarma, D. Ag–NHC anchored on silica: An efficient ultra-low loading catalyst for regioselective 1,2,3-triazole synthesis. New J. Chem. 2019, 43, 19331–19337. [Google Scholar] [CrossRef]
- Antony, A.M.; Kandathil, V.; Kempasiddaiah, M.; Sasidhar, B.; Patil, S.A.; Patil, S.A. Hexagonal Boron Nitride Supported N-Heterocyclic Carbene-Palladium(II): A New, Efficient and Recyclable Heterogeneous Catalyst for Suzuki-Miyaura Cross-Coupling Reaction. Catal. Lett. 2021, 151, 1293–1308. [Google Scholar] [CrossRef]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, R.; Tantayanon, S.; Bag, B.G. Synthesis of palladium nanoparticles with leaf extract of Chrysophyllum cainito (Star apple) and their applications as efficient catalyst for C–C coupling and reduction reactions. Int. Nano Lett. 2017, 7, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Kandathil, V.; Dateer, R.B.; Sasidhar, B.; Patil, S.A.; Patil, S.A. Green synthesis of palladium nanoparticles: Applications in aryl halide cyanation and Hiyama cross-coupling reaction under ligand free conditions. Catal. Lett. 2018, 148, 1562–1578. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: Catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. Adv. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.M.; Yelamaggad, C.; Patil, S.A. Palladium nanoparticles decorated on functionalized graphitic carbon nitride as an efficient and retrievable nanocatalyst for organic dye degradation and hydrogen peroxide sensing. Mater. Chem. Phys. 2023, 297, 127370. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, B.; Liu, H.; Liu, Z.; Zhang, X.; Zheng, X.; Liu, Q. FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sens. Actuators B Chem. 2018, 259, 775–783. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, R.; Chen, W. Photochemical deposition of surface-clean silver nanoparticles on nitrogen-doped graphene quantum dots for sensitive colorimetric detection of glutathione. Sens. Actuators B Chem. 2016, 228, 66–73. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, H.; Dai, S.; Zhi, X.; Zhang, J.; Li, W. Glutathione-stabilized palladium nanozyme for colorimetric assay of silver(I) ions. Analyst 2015, 140, 6676–6683. [Google Scholar] [CrossRef]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res. Int. 2013, 2013, 329121. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A. Utilization of the peroxidase-like activity of silver nanoparticles nanozyme on o-phenylenediamine/H2O2 system for fluorescence detection of mercury(II) ions. Sci. Rep. 2022, 12, 6953. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodoro, K.B.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Zhu, X.-Y.; Jiao, H.-J.; Dong, Y.-M.; Li, Z.-J. Ultrasensitive and dual functional colorimetric sensors for mercury(II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens. Bioelectron. 2012, 31, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.-C.; Nam, Y.-S.; Lee, H.-J.; Lee, K.-B. A colorimetric probe to determine Pb2+ using functionalized silver nanoparticles. Analyst 2015, 140, 8209–8216. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.j.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B. 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S.; Sethi, D.; Kumar, C.G. Comparative evaluation of synthetic routes and antibacterial/antifungal properties of Zn–Al layered double hydroxides containing benzoate anion. Environ. Eng. Sci. 2018, 35, 247–260. [Google Scholar] [CrossRef]
- Marcato, P.D.; Parizotto, N.V.; Martinez, D.S.T.; Paula, A.J.; Ferreira, I.R.; Melo, P.S.; Durán, N.; Alves, O.L. New hybrid material based on layered double hydroxides and biogenic silver nanoparticles: Antimicrobial activity and cytotoxic effect. J. Braz. Chem. Soc. 2013, 24, 266–272. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Potheher, I.V. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. New J. Chem. 2017, 41, 10347–10356. [Google Scholar] [CrossRef]
- Devadiga, A.; Shetty, K.V.; Saidutta, M. Timber industry waste-teak (Tectona grandis Linn.) leaf extract mediated synthesis of antibacterial silver nanoparticles. Int. Nano Lett. 2015, 5, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Ogunmefun, O.T.; Ekundayo, E.A.; Akharaiyi, F.C.; Ewhenodere, D. Phytochemical screening and antibacterial activities of Tectona grandis L. f. (Teak) leaves on microorganisms isolated from decayed food samples. Trop. Plant Res. 2017, 4, 376–382. [Google Scholar] [CrossRef]
- Vyas, P.; Yadav, D.K.; Khandelwal, P. Tectona grandis (teak)–A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2019, 33, 2338–2354. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Yanming, S.; Dongbin, L.; Shifeng, L.; Lihui, F.; Shuai, C.; Haque, M.A. Removal of lead from aqueous solution on glutamate intercalated layered double hydroxide. Arab. J. Chem. 2017, 10, S2295–S2301. [Google Scholar] [CrossRef] [Green Version]
- Ay, A.N.; Zümreoglu-Karan, B.; Mafra, L. A Simple Mechanochemical Route to Layered Double Hydroxides: Synthesis of Hydrotalcite-Like Mg-Al-NO3-LDH by Manual Grinding in a Mortar. Z. Anorg. Allg. Chem. 2009, 635, 1470–1475. [Google Scholar] [CrossRef]
- Bontchev, R.P.; Liu, S.; Krumhansl, J.L.; Voigt, J.; Nenoff, T.M. Synthesis, characterization, and ion exchange properties of hydrotalcite Mg6Al2(OH)16(A)x(A‘)2−x⊙4H2O (A, A‘ = Cl−, Br−, I−, and NO3−, 2 ≥ x ≥ 0) derivatives. Chem. Mater. 2003, 15, 3669–3675. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ramakrishnappa, T. Green synthesized uncapped Ag colloidal nanoparticles for selective colorimetric sensing of divalent Hg and H2O2. J. Environ. Chem. Eng. 2021, 9, 105365. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S. Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: Catalytic properties of the resulting particles. J. Colloid Interface Sci. 2016, 462, 243–251. [Google Scholar] [CrossRef]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of phosphate from industrial wastewater using uncalcined MgAl-NO3 layered double hydroxide: Batch study and modeling. Desalin. Water Treat. 2016, 57, 15920–15931. [Google Scholar] [CrossRef]
- Govindarajan, M.; Hoti, S.; Rajeswary, M.; Benelli, G. One-step synthesis of polydispersed silver nanocrystals using Malva sylvestris: An eco-friendly mosquito larvicide with negligible impact on non-target aquatic organisms. Parasitol. Res. 2016, 115, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liang, X.; Zhao, T.; Hu, Y.; Zhu, P.; Sun, R. Facile synthesis of monodisperse silver nanoparticles for screen printing conductive inks. J. Mater. Sci. Mater. Electron. 2017, 28, 16939–16947. [Google Scholar] [CrossRef]
- Han, L.; Li, C.; Zhang, T.; Lang, Q.; Liu, A. Au@Ag heterogeneous nanorods as nanozyme interfaces with peroxidase-like activity and their application for one-pot analysis of glucose at nearly neutral pH. ACS Appl. Mater. Interfaces 2015, 7, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, Z. Fluorescent and colorimetric dual detection of mercury(II) by H2O2 oxidation of o-phenylenediamine using Pt nanoparticles as the catalyst. Sens. Actuators B Chem. 2017, 249, 53–58. [Google Scholar] [CrossRef]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Pavunraj, M.; Annadurai, G. Green synthesis of silver nanoparticle and silver based chitosan bionanocomposite using stem extract of Saccharum officinarum and assessment of its antibacterial activity. Adv. Nat. Sci. Nanosci. 2017, 8, 035019. [Google Scholar] [CrossRef]
- Spadaro, J.; Berger, T.; Barranco, S.; Chapin, S.; Becker, R. Antibacterial effects of silver electrodes with weak direct current. Antimicrob. Agents Chemother. 1974, 6, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, H.; Zhao, Q.; Xing, L.; Zheng, X.; Wang, W.; Zhao, Y.; Yu, Y.; Hu, L.; Yao, W. Recent developments of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst 2020, 145, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Rana, J.; Jamdagni, P.; Sindhu, A. Phytochemical screening, GC-MS and FT-IR analysis of methanolic extract leaves of Elettaria cardamomum. Int. J. Res. 2017, 5, 213–224. [Google Scholar] [CrossRef]














| Sample | Mean ZOI Observed in Case of E. coli | Mean ZOI Observed in Case of B. cereus | ||||
|---|---|---|---|---|---|---|
| Ciprofloxacin | 27 mm | 24 mm | ||||
| Mg-Al-OH | 30 μg | 60 μg | 90 μg | 30 μg | 60 μg | 90 μg |
| 3 mm | 6 mm | 8 mm | - | 2 mm | 4.5 mm | |
| Mg-Al-OH@TGLE-AgNPs | 30 μg | 60 μg | 90 μg | 30 μg | 60 μg | 90 μg |
| 8 mm | 12 mm | 14 mm | 8 mm | 9 mm | 18 mm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamanmalik, M.I.; Antony, A.M.; Yelamaggad, C.V.; Patil, S.A.; Patil, S.A. Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity. Molecules 2023, 28, 5754. https://doi.org/10.3390/molecules28155754
Chamanmalik MI, Antony AM, Yelamaggad CV, Patil SA, Patil SA. Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity. Molecules. 2023; 28(15):5754. https://doi.org/10.3390/molecules28155754
Chicago/Turabian StyleChamanmalik, Masira I., Arnet Maria Antony, C. V. Yelamaggad, Shivaputra A. Patil, and Siddappa A. Patil. 2023. "Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity" Molecules 28, no. 15: 5754. https://doi.org/10.3390/molecules28155754
APA StyleChamanmalik, M. I., Antony, A. M., Yelamaggad, C. V., Patil, S. A., & Patil, S. A. (2023). Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity. Molecules, 28(15), 5754. https://doi.org/10.3390/molecules28155754