Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration
Abstract
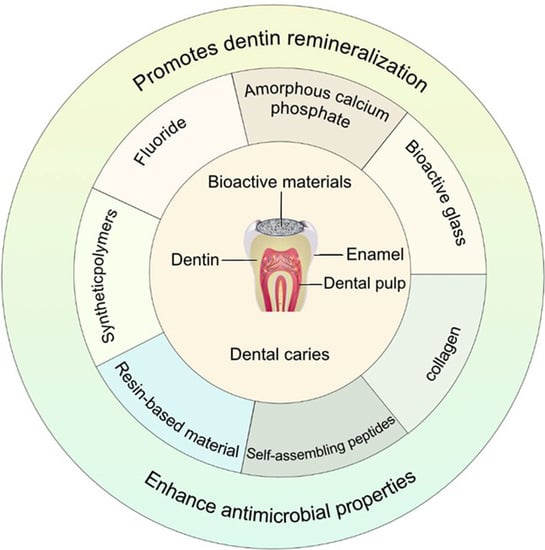
:1. Introduction
2. Fluoride
3. Amorphous Calcium Phosphate
4. Bioactive Glass
5. Collagen
6. Self-Assembling Peptides
7. Resin-Based Material
8. Synthetic Polymers
9. Conclusions and Outlook
- The filling must establish complete adhesion to the tooth surface to prevent leakage.
- The filling must possess adequate compressive strength to withstand the forces exerted during mastication.
- The filling must be biocompatible and not pose toxicity risks to the surrounding tooth tissues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental Caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Martins-Júnior, P.A.; Vieira-Andrade, R.G.; Corrêa-Faria, P.; Oliveira-Ferreira, F.; Marques, L.S.; Ramos-Jorge, M.L. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. 2013, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.A.; Fan, M.W. Research progress in ecological prevention of dental caries. Chin. J. Stomatol. 2022, 57, 297–301. [Google Scholar]
- Gao, Y.B.; Hu, T.; Zhou, X.D.; Shao, R.; Cheng, R.; Wang, G.S.; Yang, Y.M.; Li, X.; Yuan, B.; Xu, T.; et al. Dental Caries in Chinese Elderly People: Findings from the 4th National Oral Health Survey. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. (CSA) 2018, 21, 213–220. [Google Scholar]
- Balaji, S.M. Economic impact of dental caries in India. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2018, 29, 132. [Google Scholar] [CrossRef]
- Parhi, S.; Pal, S.; Das, S.K.; Ghosh, P. Strategies toward development of antimicrobial biomaterials for dental healthcare applications. Biotechnol. Bioeng. 2021, 118, 4590–4622. [Google Scholar] [CrossRef]
- Gallusi, G.; Libonati, A.; Piro, M.; Di Taranto, V.; Montemurro, E.; Campanella, V. Is Dental Amalgam a Higher Risk Factor rather than Resin-Based Restorations for Systemic Conditions? A Systematic Review. Materials 2021, 14, 1980. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Worthington, H.V.; Khangura, S.; Seal, K.; Mierzwinski-Urban, M.; Veitz-Keenan, A.; Sahrmann, P.; Schmidlin, P.R.; Davis, D.; Iheozor-Ejiofor, Z.; Alcaraz, M.G.R. Direct composite resin fillings versus amalgam fillings for permanent posterior teeth. Cochrane Database Syst. Rev. 2021, 8, Cd005620. [Google Scholar]
- He, L.; Hao, Y.; Zhen, L.; Liu, H.; Shao, M.; Xu, X.; Liang, K.; Gao, Y.; Yuan, H.; Li, J.; et al. Biomineralization of dentin. J. Struct. Biol. 2019, 207, 115–122. [Google Scholar] [CrossRef]
- Chisini, L.A.; Collares, K.; Cademartori, M.G.; de Oliveira, L.J.C.; Conde, M.C.M.; Demarco, F.F.; Corrêa, M.B. Restorations in primary teeth: A systematic review on survival and reasons for failures. Int. J. Paediatr. Dent. 2018, 28, 123–139. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Mei, M.L.; Lo, E.C.M. Use of fluorides in dental caries management. Gen. Dent. 2010, 58, 37–43; quiz 44–45, 79–80. [Google Scholar] [PubMed]
- Kawasaki, K.; Ruben, J.; Stokroos, I.; Takagi, O.; Arends, J. The remineralization of EDTA-treated human dentine. Caries Res. 1999, 33, 275–280. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, W.; Ning, T.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. A novel oligopeptide simulating dentine matrix protein 1 for biomimetic mineralization of dentine. Clin. Oral Investig. 2014, 18, 873–881. [Google Scholar] [CrossRef]
- Nimbeni, S.B.; Nimbeni, B.S.; Divakar, D.D. Role of Chitosan in Remineralization of Enamel and Dentin: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2021, 14, 562–568. [Google Scholar] [CrossRef]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, Cd007868. [Google Scholar] [CrossRef]
- Shindova, M. Root canal filling materials in primary teeth—Review. Folia Med. 2021, 63, 657–662. [Google Scholar] [CrossRef]
- Bijle, M.N.A.; Ekambaram, M.; Lo, E.C.; Yiu, C.K.Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 2018, 76, 75–82. [Google Scholar] [CrossRef]
- Chen, Y.D.; Shu, C.; Duan, Z.H.; Xu, J.J.; Li, X.J.; Chen, F.; Luo, Q.J. Synthesis and characterization of an anti-caries and remineralizing fluorine-containing cationic polymer PHMB-F. Biomater. Sci. 2021, 9, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-Q.; Zhu, Y.-J.; Chen, F.-F.; Jiang, Y.-Y.; Xiong, Z.-C.; Chen, F. Antibacterial gluey silver-calcium phosphate composites for dentine remineralization. J. Mater. Chem. B 2018, 6, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yang, J.; Xu, H.H.K.; Weir, M.D.; Tao, S.; Yu, Z.; Liu, Y.; Li, M.; Zhou, X.; Liang, K.; et al. Remineralization effectiveness of adhesive containing amorphous calcium phosphate nanoparticles on artificial initial enamel caries in a biofilm-challenged environment. Clin. Oral Investig. 2021, 25, 5375–5390. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; dos Passos Silva, M.; Cannon, M.L.; Danelon, M. Combined effect of casein phosphopeptide-amorphous calcium phosphate and sodium trimetaphosphate on the prevention of enamel demineralization and dental caries: An in vitro study. Clin. Oral Investig. 2021, 25, 2811–2820. [Google Scholar] [CrossRef]
- Fernando, J.R.; Shen, P.; Sim, C.P.C.; Chen, Y.-Y.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Stanton, D.P.; MacRae, C.M.; Reynolds, E.C. Self-assembly of dental surface nanofilaments and remineralisation by SnF2 and CPP-ACP nanocomplexes. Sci. Rep. 2019, 9, 1285. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Li, M.; Li, Y.; Pang, Y.; Deng, J.; Zhang, X.; Liu, W. Polyzwitterion Manipulates Remineralization and Antibiofilm Functions against Dental Demineralization. ACS Nano 2022, 16, 3119–3134. [Google Scholar] [CrossRef]
- Wu, Q.; Mei, M.L.; Wu, X.; Shi, S.; Xu, Y.; Chu, C.H.; Chen, Y. Remineralising effect of 45S5 bioactive glass on artificial caries in dentine. BMC Oral Health 2020, 20, 49. [Google Scholar] [CrossRef]
- Rodriguez, O.; Alhalawani, A.; Arshad, S.; Towler, M.R. Rapidly-Dissolving Silver-Containing Bioactive Glasses for Cariostatic Applications. J. Funct. Biomater. 2018, 9, 28. [Google Scholar] [CrossRef]
- Da Silva Meirelles Dória Maia, J.N.; Portela, M.B.; Candela, D.R.S.; Neves, A.D.A.; Noronha-Filho, J.D.; Mendes, A.D.O.; Barros, M.A.; da Silva, E.M. Fabrication and characterization of remineralizing dental composites containing calcium type pre-reacted glass-ionomer (PRG-Ca) fillers. Dent. Mater. 2021, 37, 1325–1336. [Google Scholar] [CrossRef]
- Tao, S.; Yang, J.; Su, Z.; Zhou, F.; Wang, Z.; Yang, Y.; Sun, L.; Deng, Y.; Liang, K.; Li, J. A Dentin Biomimetic Remineralization Material with an Ability to Stabilize Collagen. Small 2022, 18, 2203644. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Qin, H.; Long, J.; Lin, X.; Xie, F. Remineralization of human dentin type I collagen fibrils induced by carboxylated polyamidoamine dendrimer/amorphous calcium phosphate nanocomposite: An in vitro study. J. Biomater. Sci. Polym. Ed. 2022, 33, 668–686. [Google Scholar] [CrossRef]
- Jin, W.; Jin, Y.; Duan, P.; Wu, H.; Zhang, L.; Du, Q.; Pan, H.; Tang, R.; Shao, C. Promotion of collagen mineralization and dentin repair by succinates. J. Mater. Chem. B 2022, 10, 5826–5834. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Du, Q.; Qu, Y.; Shao, C.; Chen, C.; Sun, J.; Mao, C.; Tang, R.; Gu, X. Tannic acid induces dentin biomineralization by crosslinking and surface modification. RSC Adv. 2022, 12, 3454–3464. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ding, L.; Wang, Y.; Han, S.; Zheng, S.; Guo, Q.; Li, W.; Zhou, X.; Zhang, L. The effects of 8DSS peptide on remineralization in a rat model of enamel caries evaluated by two nondestructive techniques. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827798. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Han, S.; Peng, X.; Wang, K.; Zheng, S.; Li, H.; Niu, Y.; Li, W.; Zhang, L. Tuftelin-derived peptide facilitates remineralization of initial enamel caries in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ding, L.; Li, Z.; Wang, X.; Wang, K.; Han, S.; Li, W.; Zhou, X.; Zhang, L. Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 2019, 100, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Wang, K.; Ren, Q.; Li, H.; Zheng, S.; Niu, Y.; Zhou, X.; Li, W.; Zhang, L. Bifunctional anticaries peptides with antibacterial and remineralizing effects. Oral Dis. 2019, 25, 488–496. [Google Scholar] [CrossRef]
- Yue, S.; Wu, J.; Zhang, Q.; Zhang, K.; Weir, M.D.; Imazato, S.; Bai, Y.; Xu, H.H. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J. Dent. 2018, 75, 48–57. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef]
- Abuna, G.; Campos, P.; Hirashi, N.; Giannini, M.; Nikaido, T.; Tagami, J.; Sinhoreti, M.A.C.; Geraldeli, S. The ability of a nanobioglass-doped self-etching adhesive to re-mineralize and bond to artificially demineralized dentin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, 120–130. [Google Scholar] [CrossRef]
- Mitwalli, H.; AlSahafi, R.; Albeshir, E.G.; Dai, Q.; Sun, J.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.; Weir, M.D. Novel Nano Calcium Fluoride Remineralizing and Antibacterial Dental Composites. J. Dent. 2021, 113, 103789. [Google Scholar] [CrossRef]
- Li, A.; Cui, Y.; Gao, S.; Li, Q.; Xu, L.; Meng, X.; Dong, Y.; Liu, X.; Qiu, D. Biomineralizing Dental Resin Empowered by Bioactive Amphiphilic Composite Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, J.; Hu, B.; Li, Z.; Wu, M.; Guo, H.; Huang, X.; Liu, X.; Guo, X.; Liu, P.; et al. Amyloid-Mediated Remineralization in Pit and Fissure for Caries Preventive Therapy. Adv. Healthc. Mater. 2022, 11, 2200872. [Google Scholar] [CrossRef]
- Xu, X.; Wang, N.; Wu, M.; Wang, J.; Wang, D.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Programmed antibacterial and mineralization therapy for dental caries based on zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid material. Colloids Surf. B Biointerfaces 2020, 194, 111206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; You, Y.; Yi, L.; Wu, X.; Zhao, Y.; Yu, J.; Liu, H.; Shen, Y.; Guo, J.; Huang, C. Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact. Mater. 2023, 20, 418–433. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm: The significance of pH in health and caries. Compend. Contin. Educ. Dent. 2009, 30, 76–78, 80, 83–87; quiz 88, 90. [Google Scholar] [PubMed]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.; Sone, E.D. In vitro models of collagen biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef]
- Weyant, R.J.; Tracy, S.L.; Anselmo, T.; Beltrán-Aguilar, E.D.; Donly, K.J.; Frese, W.A.; Hujoel, P.P.; Iafolla, T.; Kohn, W.; Kumar, J.; et al. Topical fluoride for caries prevention. J. Am. Dent. Assoc. 2013, 144, 1279–1291. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, X.; Wang, L.; Qiu, W.; Wang, S.; Zhou, Y.; Li, M.; Li, Y.; Cheng, L.; Li, J.; et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 2015, 94, 344–353. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef] [PubMed]
- Soares-Yoshikawa, A.L.; Varanda, T.; Iwamoto, A.S.; Kantovitz, K.R.; Puppin-Rontani, R.M.; Pascon, F.M. Fluoride release and remineralizing potential of varnishes in early caries lesions in primary teeth. Microsc. Res. Tech. 2021, 84, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Punhagui, M.F.; Jussiani, E.I.; Andrello, A.C.; Favaro, J.C.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Effect of application time and concentration of silver diamine fluoride on the enamel remineralization. J. Clin. Exp. Dent. 2021, 13, e653–e658. [Google Scholar] [CrossRef]
- Liao, Y.; Brandt, B.W.; Li, J.; Crielaard, W.; Van Loveren, C.; Deng, D.M. Fluoride resistance in Streptococcus mutans: A mini review. J. Oral Microbiol. 2017, 9, 1344509. [Google Scholar] [CrossRef]
- Huang, X.; Exterkate, R.A.M.; ten Cate, J.M. Factors Associated with Alkali Production from Arginine in Dental Biofilms. J. Dent. Res. 2012, 91, 1130–1134. [Google Scholar] [CrossRef]
- Divaris, K.; Preisser, J.S.; Slade, G.D. Surface-specific efficacy of fluoride varnish in caries prevention in the primary dentition: Results of a community randomized clinical trial. Caries Res. 2013, 47, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhu, Y.-J.; Zhao, X.-Y.; Lu, B.-Q.; Tang, Q.-L.; Zhao, J.; Chen, F. Highly stable amorphous calcium phosphate porous nanospheres: Microwave-assisted rapid synthesis using ATP as phosphorus source and stabilizer, and their application in anticancer drug delivery. Chemistry 2013, 19, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef]
- Weir, M.D.; Chow, L.C.; Xu, H.H. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef]
- Sodata, P.; Juntavee, A.; Juntavee, N.; Peerapattana, J. Optimization of Adhesive Pastes for Dental Caries Prevention. AAPS PharmSciTech 2017, 18, 3087–3096. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Reynolds, E.C. Calcium Phosphopeptides—Mechanisms of Action and Evidence for Clinical Efficacy. Adv. Dent. Res. 2012, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Widyarman, A.S.; Udawatte, N.S.; Theodorea, C.F.; Apriani, A.; Richi, M.; Astoeti, T.E.; Seneviratne, C.J. Casein phosphopeptide-amorphous calcium phosphate fluoride treatment enriches the symbiotic dental plaque microbiome in children. J. Dent. 2021, 106, 103582. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.G.; Pessan, J.P.; Souza, J.A.S.; Moraes, J.C.S.; Delbem, A.C.B. Cyclotriphosphate associated to fluoride increases hydroxyapatite resistance to acid attack. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2553–2564. [Google Scholar] [CrossRef] [PubMed]
- Faller, R.V.; Eversole, S.L. Protective effects of SnF2—Part III. Mechanism of barrier layer attachment. Int. Dent. J. 2014, 64, 16–21. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting Dentine Caries with Silver Diamine Fluoride: What’s Behind It? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Stamford, T.C.M.; Niederman, R. Silver Diamine Fluoride: A Caries “Silver-Fluoride Bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef]
- Bakry, A.S.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. Cytotoxicity of 45S5 bioglass paste used for dentine hypersensitivity treatment. J. Dent. 2011, 39, 599–603. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, J.; Gong, M.; Liu, Q.; Zhou, H.; Wang, J.; Mei, Y. Effects of 45S5 bioactive glass on the remineralization of early carious lesions in deciduous teeth: An in vitro study. BMC Oral Health 2021, 21, 576. [Google Scholar] [CrossRef]
- Schwendicke, F.; Al-Abdi, A.; Moscardó, A.P.; Cascales, A.F.; Sauro, S. Remineralization effects of conventional and experimental ion-releasing materials in chemically or bacterially-induced dentin caries lesions. Dent. Mater. 2019, 35, 772–779. [Google Scholar] [CrossRef]
- Cao, C.Y.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.H. Methods for Biomimetic Remineralization of Human Dentine: A Systematic Review. Int. J. Mol. Sci. 2015, 16, 4615–4627. [Google Scholar] [CrossRef]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Sadr, A.; Yamashita, K.; Tagami, J. CO2 Laser Improves 45S5 Bioglass Interaction with Dentin. J. Dent. Res. 2010, 90, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Young, R.A. Implications of Atomic Substitutions and Other Structural Details in Apatites. J. Dent. Res. 1974, 53, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of Chemical Bonding at Biomaterial-Hard Tissue Interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; de Lima Oliveira, R.F.; Amorim, A.A.; Geng-Vivanco, R.; de Carvalho Panzeri Pires-de-Souza, F. Remineralization of caries-affected dentin and color stability of teeth restored after treatment with silver diamine fluoride and bioactive glass-ceramic. Clin. Oral Investig. 2022, 26, 4805–4816. [Google Scholar] [CrossRef]
- Moecke, S.E.; Silva, A.G.d.C.S.; Andrade, A.C.M.; Borges, A.B.; Torres, C.R.G. Efficacy of S-PRG filler varnishes on enamel caries remineralization. J. Dent. 2022, 119, 104074. [Google Scholar] [CrossRef]
- Forien, J.-B.; Zizak, I.; Fleck, C.; Petersen, A.; Fratzl, P.; Zolotoyabko, E.; Zaslansky, P. Water-Mediated Collagen and Mineral Nanoparticle Interactions Guide Functional Deformation of Human Tooth Dentin. Chem. Mater. 2016, 28, 3416–3427. [Google Scholar] [CrossRef]
- Niu, L.-N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.-H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- Ganss, C.; Klimek, J.; Starck, C. Quantitative analysis of the impact of the organic matrix on the fluoride effect on erosion progression in human dentine using longitudinal microradiography. Arch. Oral Biol. 2004, 49, 931–935. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The Activation and Function of Host Matrix Metalloproteinases in Dentin Matrix Breakdown in Caries Lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Zeng, S.; Quan, X.; Zhu, H.; Sun, D.; Miao, Z.; Zhang, L.; Zhou, J. Computer Simulations on a pH-Responsive Anticancer Drug Delivery System Using Zwitterion-Grafted Polyamidoamine Dendrimer Unimolecular Micelles. Langmuir 2021, 37, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Moradian-Oldak, J.; George, A. Biomineralization of Enamel and Dentin Mediated by Matrix Proteins. J. Dent. Res. 2021, 100, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, J.; Yang, G.; Pan, Z.; Ni, X.; Xu, H.; Huang, Q. Effect of pH and succinic acid on the morphology of α-calcium sulfate hemihydrate synthesized by a salt solution method. J. Cryst. Growth 2013, 374, 31–36. [Google Scholar] [CrossRef]
- Machado, A.C.; Junior, E.D.; Gomes-Filho, J.E.; Cintra, L.T.A.; Ruviére, D.B.; Zoccal, R.; Damante, C.A.; Junior, E.G.J. Evaluation of tissue reaction to Aroeira (Myracrodruon urundeuva) extracts: A histologic and edemogenic study. J. Appl. Oral Sci. 2012, 20, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Ghaffari-Bohlouli, P.; Niknezhad, S.V.; Abedi, A.; Izadifar, Z.; Mohammadinejad, R.; Varma, R.S.; Shavandi, A. Tannic acid: A versatile polyphenol for design of biomedical hydrogels. J. Mater. Chem. B 2022, 10, 5873–5912. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Crenshaw, M.A.; Linde, A. Induction and inhibition of hydroxyapatite formation by rat dentine phosphoprotein in vitro. Arch. Oral Biol. 1988, 33, 685–691. [Google Scholar] [CrossRef]
- Cross, K.J.; Huq, N.L.; Reynolds, E.C. Protein dynamics of bovine dentin phosphophoryn. J. Pept. Res. 2005, 66, 59–67. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhou, X.; Hu, S.; Zhang, S.; Wu, H. Effect of the antimicrobial decapeptide KSL on the growth of oral pathogens and Streptococcus mutans biofilm. Int. J. Antimicrob. Agents 2011, 37, 33–38. [Google Scholar] [CrossRef]
- Mai, J.; Tian, X.-L.; Gallant Jeffrey, W.; Merkley, N.; Biswas, Z.; Syvitski, R.; Douglas, S.E.; Ling, J.; Li, Y.-H. A Novel Target-Specific, Salt-Resistant Antimicrobial Peptide against the Cariogenic Pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 5205–5213. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Woolfolk, S.K.; Cloyd, A.K.; Ye, Q.; Boone, K.; Spencer, P.; Snead, M.L.; Tamerler, C. Peptide-Enabled Nanocomposites Offer Biomimetic Reconstruction of Silver Diamine Fluoride-Treated Dental Tissues. Polymers 2022, 14, 1368. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Goddon, I.; Chen, C.-M.; Senkel, H.; Hickel, R.; Stösser, L.; Heinrich-Weltzien, R.; Kühnisch, J. Are pit and fissure sealants needed in children with a higher caries risk? Clin. Oral Investig. 2010, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Reis, A.; Bortoli, G.; Patzlaft, R.; Kenshima, S.; Filho, L.E.R.; Accorinte, M.L.R.; van Dijken, J.W.V. Influence of Adhesive Systems on Interfacial Dentin Gap Formation In Vitro. Oper. Dent. 2006, 31, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Huang, X.; Pan, Q.; Zuo, C.; Huang, C.; Yang, X.; Zhao, Y. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. J. Dent. 2011, 39, 825–833. [Google Scholar] [CrossRef] [PubMed]
- LSahafi, R.; Balhaddad, A.A.; Mitwalli, H.; Ibrahim, M.S.; Melo, M.A.S.; Oates, T.W.; Xu, H.H.; Weir, M.D. Novel Crown Cement Containing Antibacterial Monomer and Calcium Phosphate Nanoparticles. Nanomaterials 2020, 10, 2001. [Google Scholar] [CrossRef]
- Tao, S.; Su, Z.; Xiang, Z.; Xu, H.H.; Weir, M.D.; Fan, M.; Yu, Z.; Zhou, X.; Liang, K.; Li, J. Nano-calcium phosphate and dimethylaminohexadecyl methacrylate adhesive for dentin remineralization in a biofilm-challenged environment. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, e316–e328. [Google Scholar] [CrossRef]
- Soares, C.J.; Rodrigues, M.D.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 31, e62. [Google Scholar] [CrossRef]
- Jokstad, A. Secondary caries and microleakage. Dent. Mater. 2016, 32, 11–25. [Google Scholar] [CrossRef]
- Yang, B.; Guo, J.; Huang, Q.; Heo, Y.; Fok, A.; Wang, Y. Acoustic properties of interfacial debonding and their relationship with shrinkage stress in Class-I restorations. Dent. Mater. 2016, 32, 742–748. [Google Scholar] [CrossRef]
- Tavangar, M.S.; Safarpour, A.; Parizi, A.T.; Shafiei, F. Evaluating the shear bond strength and remineralization effect of calcium silicate-based and conventional self-adhesive resin cements to caries-affected dentin. Clin. Exp. Dent. Res. 2022, 8, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Fulgêncio, R.; Watson, T.F.; Cama, G.; Thompson, I.; Toledano, M. Remineralisation properties of innovative light-curable resin-based dental materials containing bioactive micro-fillers. J. Mater. Chem. B 2013, 1, 2624–2638. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, J.; Indiran, M.A.; Kumar, P.; Dooraikannan, S.; Jeevanandan, G. Microleakage Assessment of Two Different Pit and Fissure Sealants: A Comparative Confocal Laser Scanning Microscopy Study. Int. J. Clin. Pediatr. Dent. 2020, 13, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Rompante, P.; Henriques, B.; Silva, F.S.; Özcan, M.; Souza, J.C.M. Degradation of Tooth Occlusal Fissure and Pit Sealants by Wear and Corrosion Pathways: A Short Review. J. Bio- Tribo-Corros. 2021, 7, 111. [Google Scholar] [CrossRef]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, D.; Mann, S.; Roveri, N. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2007, 17, 2180–2188. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.M.; Rice, K.C.; Li, X.; Yu, F.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Tooth-binding micelles for dental caries prevention. Antimicrob. Agents Chemother. 2009, 53, 4898–4902. [Google Scholar] [CrossRef]




| Type | Name | Element | Characteristic | Advantage | Disadvantage | Result | Reference |
|---|---|---|---|---|---|---|---|
| Fluoride | Arg-NaF toothpaste | Arginine, NaF | Arginine: NaF = 1:3 | Increase the concentration of fluoride ions in clean toothpaste | As the arginine concentration increases, the pH of the toothpaste gradually decreases | NaF toothpaste significantly increased the remineralization of incipient enamel caries | [20] |
| Polyhexamethylene biguanide-AgF | Polyhexamethylene biguanide, AgF | PHMB-F has stronger antibacterial activity | PHMB-F has stronger antibacterial activity | The long-term safety of PHMB-F in vivo is unknown | PHMB-F has good antibacterial and remineralization capabilities | [21] | |
| Amorphous calcium phosphate | Gluey silver–calcium phosphate (GSCP) | GSCP, ACP | GSCP stabilizing the ACP phase and providing binding sites for Ag ions | GSCP releasing calcium ions and phosphates provides the basis for remineralization | GSCP is a jelly-like structure that does not adhere stably to the tooth’s surface | GSCP has good antibacterial and remineralization capabilities | [22] |
| NACP binder nanoparticle | Binder, ACP | The average particle size of NACP was 116 nm | The ACP binder-treated enamel showed the best remineralization | The released calcium and phosphorus ions are only effective in situ. | The ACP binder showed a remineralization value of 52.29 ± 4.79% | [23] | |
| CPP-ACP | CPP, ACP, Sodium trimetaphosphate | - | CPP-ACP decrease in mineral loss on the surface of the teeth | - | CPP-ACP can reduce the ability to demineralize teeth | [24] | |
| CPP-ACP -SnF2 | Stannous fluoride, CPP, ACP | SnF2 is remineralized by substituting hydroxyl groups in hydroxyapatite | SnF2 also enhances the ability of the mixture to release fluoride ions | - | SnF2+CPP-ACP could increase the degree of remineralization by 32% on the tenth | [25] | |
| PCBAA/ACP nanocomposites | PCBAA, ACP | PCBAA/ACP nanocomposite has an average particle size of 50.67 ± 2.37 nm | PCBAA/ACP nanocomposites can efficiently enter the dentin tubules for deep remineralization. | - | PCBAA/ACP nanocomposites has good antibacterial and remineralization capabilities | [26] | |
| 45S5 BAG | 45S5 BAG | BAG is mainly embedded in the dentin tubules to form deposits | Compared with the CPP-ACP, BAG can lead to deep remineralization | - | BAG has good remineralization capabilities | [27] | |
| BAG-silver oxide | BAG, silver oxide | The average particle size of BAG is about 4–5 μm | Silver oxide promotes the release of more cations | Silver oxide reduces the release of silicon ions. | BAG-silver oxide has good remineralization | [28] | |
| PRG | calcium fluoride, pre-reacted glass-ionomer (PRG) | The average diameter of PRG was 4.89 μm | PRG has calcium ions released and the stronger the remineralization ability | High calcium fluoride concentrations will affect composites’ physical and chemical properties | PRG enhance BAG’s remineralization capacity | [29] | |
| Collagen | PAMAM-MMP inhibitors peptide | PAMAM, Galardin | The average diameter of PAMAM-peptide was 16.8 nm | The carboxyl groups in PAMAM attract ACP to be deposited on the collagen scaffold | - | PAMAM-peptide can effectively fight dentin caries in rats | [30] |
| ACP -PAMAM | PAMAM, ACP | - | PAMAM/ACP has stronger the remineralization | PAMAM/ACP showed superior remineralization capacity of human dentin type I collagen fibers | [31] | ||
| Succinic acid -modified collagen | Succinic acid, collagen fiber | SA-modified collagen fiber scaffolds can increase the mineralization rate | SA-modified collagen did not significantly improve the mechanical properties | SA-modified collagen fiber scaffolds can increase the mineralization rate | [32] | ||
| Cross-linking collagen | Tannic acid, collagen | The degree of cross-linking of TA to collagen was 41.28 ± 1.52 | The polymer enhanced its resistance to collagenase | The self-assembly process of TA and polymer is affected by pH | Cross-linking collagen has good remineralization | [33] | |
| Self-assembling peptides | 8DSS | 8DSS | 8DSS chain can interact effectively with Ca2+ | 8DSS chain can interact effectively with Ca2+ | The remineralized layer formed by 8DSS meets the hardness of oral chewing is unknown | 8DSS peptides prevent the leaching of calcium and phosphate ions | [34] |
| Tuftelin-derived peptide | Tuftelin-derived peptide | - | TDP is a non-amelogenin protein that is deposited mainly at the dentin-enamel junction. | - | TDP has been shown to have the ability to induce remineralizatio | [35] | |
| Chitosan-QP5 (an amelogenin-derived peptide) | Chitosan, QP5 | The inhibition rate of CS-QP5 hydrogel is as high as 95.43% | CS-QP5 has demonstrated its dual antimicrobial and remineralizing effects in vitro | the safety of CS in vivo is unknown | Chitosan-QP5 has been shown to have the ability to induce remineralizatio | [36] | |
| TD7 | - | TD7 has been shown to bind to calcium ions and stabilize HA vigilance structures | TD7 has been shown to bind to calcium ions and stabilize HA vigilance structures | - | TD7 peptides effectively inhibit biofilm formation, shallower lesion depth, and higher mineral content. | [37] | |
| Resin-based material | PUF-DMAHDM-ACP resin binder | PUF, DMAHDM, ACP | 7.5% PUF in 70 μm microplastic granules and added them to the adhesive together with 10% DMAHDM and 20% ACP | PUF-DMAHDM-ACP resin binder can neutralize bacterial acids, kill bacteria, and achieve remineralization | PUF-DMAHDM-ACP resin binder inhibit 95% of bacteria in biofilms and reduce the amount of lactic acid | [38] | |
| TEG-DVBE- UDMA | TEG, DVBE, UDMA | UDMA added to TEG-DVBE increases conversion and intensity | UDMP did not affect the mechanical properties of the composite | TEG-DVBE- UDMA increasing dentin hardness by up to 41% compared to commercial fluoride | [39] | ||
| BAG nanoparticles resin | BAG, 0.05–2 μm nanoparticles, calcium silicate resin | BAG synthesized into 0.05–2 μm nanoparticles and incorporated them into a calcium silicate resin | composite resins for remineralization and antimicrobial power | BAG nanoparticles resin withstand the challenge of higher hardness for 28 days. | [40] | ||
| calcium fluoride- ACP-DMAHDM resin | Calcium, ACP, DMAHDM resin | BAG fillers are hydrophilic and incompatible with hydrophobic resin matrices | calcium fluoride- ACP-DMAHDM resin releases high fluoride and calcium ions to induce remineralization | BAG fillers tend to aggregate in the resin matrix, may lead to a serious deterioration in the mechanical properties | calcium fluoride- ACP-DMAHDM resin flexural strength was 125.93 ± 7.49 MPa, within the recommended range of ISO | [41] | |
| BRP-containing resin composites | BAG, BRPs | BRP has a diameter of 100 nanometers resin composites has amphiphilic surface properties | BRP-containing resin composite particles have good mechanical properties, water adsorption resistance, and solubility | BRP-containing resin composites have better mechanical properties, water adsorption resistance, and solubility | [42] | ||
| Synthetic polymers | lysozyme-PEG nanoparticles | Lysozyme, polyethylene glycol (PEG) | lysozyme-PEG nanoparticles penetrate to 20,000 μm in 180 s and form a nanomembrane | lysozyme-PEG has strong interfacial bonding stability, antibacterial ability and good biocompatibility | lysozyme-PEG has deep adhesion and remineralization with simple application or gargle | [43] | |
| ZHA@ALN-PAA | PAA, ALN, ZHA | simulate NCP | ZHA@ALN-PAA can adsorbs and releases phosphate ions to promote remineralization | ZHA@ALN-PAA can simulated oral environments and form nanorod structures for remineralization | [44] | ||
| PMs@NaF-SAP | PM3, SAP, NaF | PMs@NaF-SAP has antibacterial effect and remineralization ability | PMs@NaF-SAP can remove contents to achieve antimicrobial and remineralization effects | PMs@NaF-SAP has clinical translational potiential in redent caries models | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, M.; Xue, M.; Kang, Y.; Liu, D.; Wen, Y.; Zhao, D.; Guan, B. Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration. Molecules 2023, 28, 6373. https://doi.org/10.3390/molecules28176373
Li Y, Liu M, Xue M, Kang Y, Liu D, Wen Y, Zhao D, Guan B. Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration. Molecules. 2023; 28(17):6373. https://doi.org/10.3390/molecules28176373
Chicago/Turabian StyleLi, Yuexiao, Minda Liu, Mingyu Xue, Yuanyuan Kang, Dongjuan Liu, Yan Wen, Duoyi Zhao, and Boyu Guan. 2023. "Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration" Molecules 28, no. 17: 6373. https://doi.org/10.3390/molecules28176373
APA StyleLi, Y., Liu, M., Xue, M., Kang, Y., Liu, D., Wen, Y., Zhao, D., & Guan, B. (2023). Engineered Biomaterials Trigger Remineralization and Antimicrobial Effects for Dental Caries Restoration. Molecules, 28(17), 6373. https://doi.org/10.3390/molecules28176373