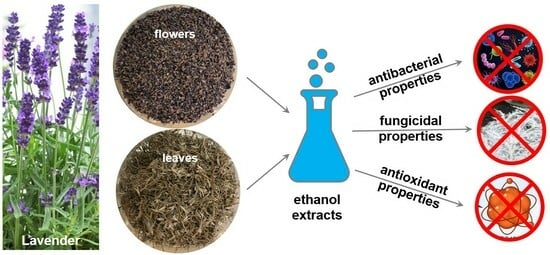
Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity
2.2. Antioxidant Assay
2.3. Chemical Composition
2.4. Graphical Interpretation of Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
Preparation of Ethanol Extracts
4.2. Methods
4.2.1. Evaluation of Fungicidal Properties against Mold Fungi on a Malt-Agar Medium
4.2.2. Evaluation of Bactericidal and Anti-Yeast Properties
4.2.3. Antioxidants Properties
4.2.4. GC-MS Analysis
4.2.5. Phenolic Compound Identification
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of Lavender (Lavandula vera L.) for Phytoremediation of Soils Contaminated with Heavy Metals. Int. J. Sci. Res. Innov. 2015, 9, 465–472. [Google Scholar]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef] [PubMed]
- Tăbăraşu, A.-M.; Anghelache, D.-N.; Găgeanu, I.; Biris, S.-S.; Vlădut, N.-V. Considerations on the Use of Active Compounds Obtained from Lavender. Sustainability 2023, 15, 8879. [Google Scholar] [CrossRef]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation; IntechOpen: London, UK, 2018; ISBN 978-1-78923-780-1. [Google Scholar]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation Substrate Composition Influences Morphology, Volatilome and Essential Oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Détár, E.; Németh, É.Z.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula x intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Caser, M.; Falla, N.M.; Demasi, S.; Scariot, V. From Fresh to Dried Lavender Flower: Changes in Phytochemical Profile According to Drying Method. Horticulturae 2023, 9, 700. [Google Scholar] [CrossRef]
- Zeljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E. Distillation Time Effect on Lavender Essential Oil Yield and Composition. J. Oleo. Sci. 2013, 62, 195–199. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Pisulewska, E.; Janeczko, Z. Krajowe Rośliny Olejkowe; Know-How: Kraków, Poland, 2008; p. 137. [Google Scholar]
- Pisulewska, E.; Puchalska, H.; Zaleski, T. Uprawa Lawendy Wąskolistnej (Lavandula angustifolia Mill) na Wyżynie Miechowskiej; Akademia Rolnicza w Krakowie: Kraków, Poland, 2004; pp. 1–39. [Google Scholar]
- Imane, M.M.; Houda, F.; Amal, A.H.S.; Kaotar, N.; Mohammed, T.; Imane, R.; Farid, H. Phytochemical Composition and Antibacterial Activity of Moroccan Lavandula angustifolia Mill. J. Essent. Oil-Bear. Plants 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical Profile, In Vitro Biological Activity and Comparison of Essential Oils from Fresh and Dried Flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317. [Google Scholar] [CrossRef]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal activity of essential oils against fungi isolated from air. Int. J. Occup. Environ. Health 2017, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Lahkimi, A.; Louaste, B.; Nechad, I.; Chaouch, M.; Eloutassi, N. Antibacterial, antifungal and antioxidant activity of Lavandula angustifolia of the middle atlas central (Morocco). Mor. J. Chem. 2020, 8, 905–918. [Google Scholar]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M.; Szczerbińska, D. Lavandula angustifolia Essential Oils as Effective Enhancers of Fluconazole Antifungal Activity against Candida albicans. Molecules 2023, 28, 1176. [Google Scholar] [CrossRef]
- Sen, S.; Yalcin, M. Activity of commercial still waters from volatile oils production against wood decay fungi. Maderas Cienc. Tecnol. 2010, 12, 127–133. [Google Scholar]
- Inouye, S.; Uchida, K.; Yamaguchi, H. In-vitro and in-vivo anti-Trichophyton activity of essential oils by vapour contact. Mycoses 2001, 44, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.R.; Pennec, A.P.; Nugier-Chauvin, C.; Daniellou, R.; Herrera-Estrella, L.; Chauvin, A.-L. Chemical Composition and Antibacterial Activity of Essential Oils Extracted from Plants Cultivated in Mexico. J. Mex. Chem. Soc. 2014, 58, 452–455. [Google Scholar]
- Zenao, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Di Vito, M.; Smolka, A.; Proto, M.R.; Barbanti, L.; Gelmini, F.; Napoli, E.; Bellardi, M.G.; Mattarelli, P.; Beretta, G.; Sanguinetti, M.; et al. Is the Antimicrobial Activity of Hydrolates Lower than That of Essential Oils? Antibiotics 2021, 10, 88. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. AICA 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Zych, S.; Szczerbińska, D. In vitro antibacterial effect of enrofloxacin combined with lavender essential oil on selected Salmonella serotypes isolated most commonly in poultry. Med. Weter. 2023, 79, 182–185. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.H.R.; Lai, K.S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules 2023, 28, 2943. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandin (Lavandula _ intermedia Emeric ex Loiseleur) essential oil from Spain: Determination of aromatic profile by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Nat. Prod. Res. 2016, 30, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pareek, S.; Ameta, K.D.; Sarolia, D.K.; Pilania, S.; Kaushik, R.A.; Shuklaand, K.B.; Kumari, P. Analysis of nutritional composition of sweet potato vines. Int. J. Pharm. Phytochem. 2018, 7, 104–106. [Google Scholar]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- Hanamanthagouda, M.A.; Kakkalameli, S.B.; Naik, P.M.; Nagella, P.; Seetharamareddy, H.R.; Murthy, H.N. Essential oils of Lavandula bipinnata and their antimicrobial activities. Food Chem. 2010, 118, 836–839. [Google Scholar] [CrossRef]
- Jaramillo, V.; Díaz, E.; Muñoz, L.N.; González-Barrios, A.F.; Rodríguez-Cortina, J.; Cruz, J.C.; Muñoz-Camargo, C. Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection. Pharmaceutics 2023, 15, 1739. [Google Scholar] [CrossRef]
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Effects of Lavandula angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid. Based Complement. Altern. Med. 2016, 9, 2752739. [Google Scholar] [CrossRef]
- Adaszyńska, M.; Swarcewicz, M.; Dzięcioł, M.; Dobrowolska, A. Comparison of chemical composition and antibacterial activity of lavender varieties from Poland. Nat. Prod. Res. 2013, 27, 1497–1501. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Monaco, P.; Divino, F.; Iorizzi, M.; Ranalli, G.; Fasano, F.; Saviano, G. Chemical Profiles, In Vitro Antioxidant and Antifungal Activity of Four Different Lavandula angustifolia L. EOs. Molecules 2023, 28, 392. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. Lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Vârban, D.; Zăhan, M.; Pop, C.R.; Socaci, S.; Stefan, R.; Crişan, I.; Bota, L.E.; Miclea, I.; Muscă, A.S.; Deac, A.M.; et al. Physicochemical Characterization and Prospecting Biological Activity of Some Authentic Transylvanian Essential Oils: Lavender, Sage and Basil. Metabolites 2022, 12, 962. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Snezana, A.K.; Ristivojevic, R.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar]
- Dias, I.J.; Trajano, E.R.I.S.; Castro, R.D.; Ferreira, G.L.S.; Medeiros, H.C.M.; Gomes, D.Q.C. Antifungal activity of linalool in cases of Candida spp. isolated from individuals with oral candidiasis. Braz. J. Biol. 2018, 2, 368–374. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 1993, 38, 41–43. [Google Scholar]
- Moon, T.; Cavanagh, H.M.A.; Wilkinson, J.M. Antifungal activity of Australian grown Lavandula spp. essential oils against Aspergillus nidulans, Trichophyton mentagrophytes, Leptosphaeria maculans and Sclerotinia sclerotiorum. J. Essent. Oil Res. 2007, 19, 171–175. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 5. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cebulak, T.; Oszmiański, J.; Kapusta, I.; Lchowicz, S. Effect of abiotic stress factors on polyphenolic content in the skin and flesh of pear by UPLC-PDA-Q/TOF-MS. Eur. Food Res. Technol. 2019, 245, 715–2725. [Google Scholar] [CrossRef]





| Plant Material | Concentration of Extracts in Growth Medium (mL/100 mL) | Day of Observation | p-Value | α | ||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | ||||
| Diameter of Mycelium (mm) | Tukey’s Test | |||||
| ZO1 | statistics F | 1.02 × 10−14 | 0.05 | |||
| 0 (control) | 58.8 | 90.0 | - | a | ||
| 0.5 | 35.0 | 42.0 | - | ab | ||
| 1.0 | 5.7 | 5.7 | - | b | ||
| 2.5 | 5.0 | 5.0 | - | b | ||
| 5.0 | 5.0 | 5.0 | - | b | ||
| ZO2 | statistics F | 1.67 × 10−8 | 0.05 | |||
| 0 (control) | 58.8 | 90.0 | - | a | ||
| 0.5 | 21.5 | 23.0 | - | b | ||
| 1.0 | 6.0 | 6.0 | - | b | ||
| 2.5 | 5.0 | 5.0 | - | b | ||
| 5.0 | 5.0 | 5.0 | - | b | ||
| Plant Material | Concentration of Extracts in Growth Medium (mL/100 mL) | Day of Observation | p-Value | α | ||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 7 | 9 | ||||
| Diameter of Mycelium (mm) | Tukey’s Test | |||||||
| ZO1 | statistics F | 3.81 × 10−8 | 0.05 | |||||
| 0 (control) | 16.0 | 20.3 | 28.8 | 40.2 | 44.8 | a | ||
| 0.5 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | b | ||
| 1.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| 2.5 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| ZO2 | statistics F | 9.10 × 10−8 | 0.05 | |||||
| 0 (control) | 16.0 | 20.3 | 28.8 | 40.2 | 44.8 | a | ||
| 0.5 | 5.2 | 6.3 | 6.3 | 6.7 | 6.7 | b | ||
| 1.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| 2.5 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | b | ||
| No. | Sample | Total Phenolic Content TPC | ABTS•+ RADICAL Scavenging Activity | DPPH• Radical Scavenging Activity | Ferric Reducing Antioxidant Power Assay FRAP |
|---|---|---|---|---|---|
| mg GAE/g | μmol Trolox Equivalent (TE)/g | ||||
| 1 | ZO1 | 19.78 ± 0.70 a | 168.86 ± 2.86 a | 97.47 ± 1.37 a | 62.88 ± 0.83 a |
| 2 | ZO2 | 31.64 ± 0.85 b | 274.84 ± 0.74 b | 198.21 ± 1.34 b | 110.25 ± 1.25 b |
| p-Value | X | Error | |
|---|---|---|---|
| Total phenolic content | 4.93 × 10−5 | 98.9 | 1.1 |
| ABTS•+ | 4.03 × 10−7 | 99.9 | 0.1 |
| DPPH• | 8.70 × 10−8 | 99.9 | 0.1 |
| FRAP | 6.65 × 10−7 | 99.9 | 0.1 |
| No. | RT [min] | Peak Share in the Chromatogram [%] | Ordinary Substance Name | Systematic Substance Name | No. CAS |
|---|---|---|---|---|---|
| 1 | 7.72 | 0.94 | α-Pinene | 2,6,6-Trimethylbicyclo [3.1.1]hept-2-ene | 80-56-8 |
| 2 | 8.72 | 2.39 | β-Pinene | 6,6-Dimethyl-2-methylenebicyclo [3.1.1]heptane | 127-91-3 |
| 3 | 9.04 | 4.79 | Myrcene | 7-Methyl-3-methylene-1,6-octadiene | 123-35-3 |
| 4 | 9.45 | 1.97 | 3-Carene | 3,7,7-Trimethylbicyclo [4.1.0]hept-3-ene | 13466-78-9 |
| 5 | 9.73 | 1.61 | m-Cymene (cymene isomers mix) | 1-Isopropyl-3-methylbenzene | |
| 6 | 9.83 | 7.09 | Limonene | 4-Isopropenyl-1-methyl-1-cyclohexene | 5989-27-5 |
| 7 | 10.00 | 25.81 | Ocimene isomers mix | ||
| 8 | 10.21 | 8.59 | Ocimene isomers mix | ||
| 9 | 11.16 | 2.23 | Linalool | 3,7-Dimethyl-1,6-octadien-3-ol | 78-70-6 |
| 10 | 11.36 | 2.02 | 1-Octen-3-yl acetate | 2442-10-6 | |
| 11 | 11.67 | 0.91 | 2,6-Dimethyl-2,4,6-octatriene | 673-84-7 | |
| 12 | 13.66 | 25.83 | Linalyl acetate | 3,7-Dimethyl-1,6-octadien-3-yl acetate | 115-95-7 |
| 13 | 15.43 | 0.93 | Lavandulyl acetate | 5-Methyl-2-(1-methylethenyl)-4-hexylene-1-ol acetate | 25905-14-0 |
| 14 | 16.03 | 3.99 | α-santalene | 6,7-dimethyl-7-(4-methylpent-3-enyl)-2,3,4,5-tetrahydro-1H-tricyclo [2.2.1.02,6]heptane | 512-61-8 |
| 15 | 16.09 | 10.84 | β-Caryophyllene | 8-Methylene-4,11,11-trimethylbicyclo [7.2.0]undec-4-ene | 87-44-5 |
| Substance Name | α-Pinene | β-Pinene | Myrcene | 3-Carene | m-Cymene | Limonene | Ocimene (Mix) | Ocimene (Mix) | Linalool | 1-Octen-3-yl Acetate | 2,6-Dimethyl-2,4,6-octatriene | Linalyl Acetate | Lavandulyl Acetate | α-Santalene | β-Caryophyllene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight [g/mol] | 136 | 136 | 196 | 136 | 134 | 136 | 136 | 136 | 154 | 170 | 136 | 196 | 196 | 204 | 204 |
| Calculated Kovats retention index | 933 | 964 | 991 | 1016 | 1023 | 1034 | 1050 | 1060 | 1100 | 1120 | 1130 | 1261 | 1288 | 1420 | 1425 |
| Reference Kovats retention index * | 926–1045 | 934–1138 | 986–1187 | 1004–1017 | 1010–1267 | 1031–1056 | 1043–1270 | 1043–1270 | 1082–1570 | 1097–1373 | 1129 | 1257–1569 | 1288–1597 | 1405–1574 | 1418–1657 |
| No. | RT [min] | Peak Share in the Chromatogram [%] | Ordinary Substance Name | Systematic Substance Name | No. CAS |
|---|---|---|---|---|---|
| 1 | 9.45 | 4.08 | 3-Carene | 3,7,7-Trimethylbicyclo [4.1.0]hept-3-ene | 13466-78-9 |
| 2 | 9.69 | trace | p-Cymene (cymene isomers mix) | 1-Isopropyl-4-methylbenzene | |
| 3 | 9.74 | trace | m-Cymene (cymene isomers mix) | 1-Isopropyl-3-methylbenzene | |
| 4 | 9.83 | trace | Limonene | 4-Isopropenyl-1-methyl-1-cyclohexene | 5989-27-5 |
| 5 | 9.89 | trace | Eucalyptol | 1,3,3-Trimethyl-2-oxabicyclo [2.2.2]octane | 470-82-6 |
| 6 | 14.66 | 4.70 | silane | ||
| 7 | 16.03 | 12.12 | α-santalene | 6,7-dimethyl-7-(4-methylpent-3-enyl)-2,3,4,5-tetrahydro-1H-tricyclo [2.2.1.02,6]heptane | 512-61-8 |
| 8 | 16.09 | 10.00 | β-Caryophyllene | 8-Methylene-4,11,11-trimethylbicyclo [7.2.0]undec-4-ene | 87-44-5 |
| 9 | 16.27 | 23.19 | Coumarin | 1-Benzopyran-2-one | 91-64-5 |
| 10 | 17.25 | 20.45 | γ-cadinene | 7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene | 39029-41-9 |
| 11 | 19.67 | 19.85 | 7-Methoxycoumarin | Methyl umbelliferyl ether | 531-59-9 |
| Substance Name | 3-Carene | p-Cymene | m-Cymene | Limonene | Eucalyptol | α-Santalene | β-Caryophyllene | Coumarin | γ-Cadinene | 7-Methoxycoumarin |
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight [g/mol] | 136 | 134 | 134 | 136 | 154 | 204 | 204 | 146 | 204 | 176 |
| Calculated Kovats retention index | 1016 | 1020 | 1023 | 1034 | 1050 | 1420 | 1425 | 1429 | 1513 | 1660 |
| Reference Kovats retention index * | 1004–1017 | 1016–1303 | 1010–1267 | 1031–1056 | 1025–1224 | 1405–1574 | 1418–1657 | 1428–2465 | 1512–1819 | 1660–2981 |
| No. | Compound | RT [min] | ZO1 | ZO2 | Type |
|---|---|---|---|---|---|
| [µg/g] | |||||
| 1 | Syringic acid glucoside | 1.933 | 70.15 | 328.10 | phenolic acid |
| 2 | Caftaric acid | 2.303 | 101.03 | 1110.18 | phenolic acid |
| 3 | Ferulic acid glucosie I | 3.081 | <LOQ | <LOQ | phenolic acid |
| 4 | Coumaric acid glucoside I | 3.121 | 671.26 | 797.42 | phenolic acid |
| 5 | Caffeic acid | 3.255 | 209.88 | 699.87 | phenolic acid |
| 6 | Ferulic acid glucosie II | 3.596 | 637.33 | 1156.45 | phenolic acid |
| 7 | Isorhamnetin 3-O-rutinoside | 3.66 | <LOQ | <LOQ | flavonoid |
| 8 | Apigenin 4′-O-glucoside-7-O-glucuronide | 3.829 | 146.58 | 170.02 | flavonoid |
| 9 | Coumaric acid glucoside II | 4.083 | 363.19 | 405.22 | phenolic acid |
| 10 | Chicoric acid | 4.397 | 221.68 | 79.83 | phenolic acid |
| 11 | Ferulic acid glucosie III | 4.563 | 813.23 | 1564.10 | phenolic acid |
| 12 | Isorhamnetin 3-O-rhamnoside | 4.775 | 123.10 | 1151.83 | flavonoid |
| 13 | (+)Catechin-rhamnoside-pentoside | 4.888 | 174.57 | 318.87 | flavonoid |
| 14 | Salvinic acid B | 5.305 | 87.09 | 97.26 | phenolic acid |
| 15 | Apigenin C-glucoside | 5.436 | 177.15 | 267.97 | flavonoid |
| 16 | Rosmarinic acid | 5.623 | 917.15 | 2547.31 | phenolic acid |
| 17 | Ferulic acid | 5.95 | 37.19 | 330.57 | phenolic acid |
| 18 | Unidentified caffeic acid derivative | 6.064 | 100.30 | 2826.27 | phenolic acid |
| 19 | Kaempferol | 6.696 | 92.23 | 821.82 | flavonoid |
| 20 | Undefined caffeic acid derivative | 7.036 | 102.62 | 4085.14 | phenolic acid |
| 21 | Undefined caffeic acid derivative | 7.333 | 55.05 | 131.71 | phenolic acid |
| 22 | Apigenin | 7.601 | 94.71 | 54.53 | flavonoid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betlej, I.; Andres, B.; Cebulak, T.; Kapusta, I.; Balawejder, M.; Jaworski, S.; Lange, A.; Kutwin, M.; Pisulewska, E.; Kidacka, A.; et al. Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland. Molecules 2023, 28, 6416. https://doi.org/10.3390/molecules28176416
Betlej I, Andres B, Cebulak T, Kapusta I, Balawejder M, Jaworski S, Lange A, Kutwin M, Pisulewska E, Kidacka A, et al. Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland. Molecules. 2023; 28(17):6416. https://doi.org/10.3390/molecules28176416
Chicago/Turabian StyleBetlej, Izabela, Bogusław Andres, Tomasz Cebulak, Ireneusz Kapusta, Maciej Balawejder, Sławomir Jaworski, Agata Lange, Marta Kutwin, Elżbieta Pisulewska, Agnieszka Kidacka, and et al. 2023. "Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland" Molecules 28, no. 17: 6416. https://doi.org/10.3390/molecules28176416
APA StyleBetlej, I., Andres, B., Cebulak, T., Kapusta, I., Balawejder, M., Jaworski, S., Lange, A., Kutwin, M., Pisulewska, E., Kidacka, A., Krochmal-Marczak, B., & Borysiuk, P. (2023). Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland. Molecules, 28(17), 6416. https://doi.org/10.3390/molecules28176416