A Strategy to Valorize a By-Product of Pine Wood (Pinus pinaster) for Copper Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
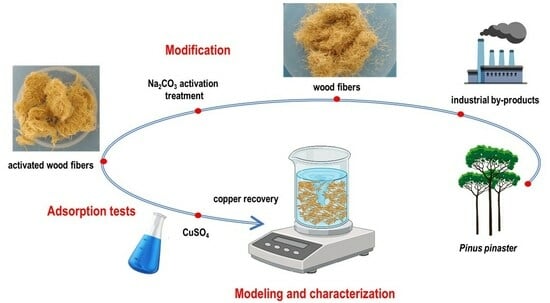
3.1. Materials
3.2. Synthetic Solutions
3.3. Experimental Protocol
3.4. Chemical Analysis
3.5. Modeling
3.6. Microscopic and Spectroscopic Characterization of Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Crini, G. Emerging Contaminants—Occurrence and Impact. Environmental Chemistry for a Sustainable World; Springer Nature: Cham, Switzerland, 2021; ISBN 978-3-030-69078-6. [Google Scholar]
- Druart, C.; Morin-Crini, N.; Euvrard, E.; Crini, G. Chemical and ecotoxicological monitoring of discharge water from a metal-finishing factory. Environ. Processes 2016, 3, 59–72. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Eaux Industrielles Contaminées—Réglementation, Paramètres Chimiques et Biologiques & Procédés d’Épuration Innovants; Presses Universitaires de Franche-Comté: Besançon, France, 2017; ISBN 978-2-84867-589-3. [Google Scholar]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D.X. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Orozco, C.I.; Freire, S.; Gόmez-Díaz, D.; González-Álvarez, J. Removal of copper from aqueous solution by biosorption onto pine sawdust. Sustain. Chem. Pharm. 2023, 32, 101016. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Green Adsorbents for Pollutant Removal. Innovative Materials. Environmental Chemistry for a Sustainable World; Springer Nature: Cham, Switzerland, 2018; ISBN 978-3-319-92162-4. [Google Scholar]
- Bashir, M.; Tyagi, S.; Annachhatre, A.P. Adsorption copper from aqueous solution onto agricultural adsorbent: Kinetics and isotherm studies. Mater. Today Proc. 2020, 28, 1833–1840. [Google Scholar] [CrossRef]
- Nagy, B.; Mânzatu, C.; Török, A.; Indolean, C.; Măicăneanu, A.; Tonk, S.; Majdik, C. Isotherm and thermodynamic studies of Cd(II) removal process using chemically modified lignocellulosic adsorbent. Rev. Roum. Chim. 2015, 60, 257–264. [Google Scholar]
- Emenike, P.C.; Omole, D.O.; Ngene, B.U.; Tenebe, I.T. Potentiality of agricultural adsorbent for the sequestering of metal ions from wastewater. Glob. J. Environ. Sci. Manag. 2016, 2, 411–442. [Google Scholar]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose based grafted biosorbents—Journey from lignocellulose biomass to toxic metal ions sorption applications—A review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost biosorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.X.; Du, X.H.; Dong, L.M. Xanthate-modified magnetic Fe3O4@SiO2-based polyvinyl alcohol/chitosan composite material for efficient removal of heavy metal ions from water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef]
- Dong, L.M.; Shan, C.Y.; Liu, Y.; Sun, H.; Yao, B.; Gong, G.Z.; Jin, X.D.; Wang, S.F. Characterization and mechanistic study of heavy metal adsorption by facile synthesized magnetic xanthate-modified chitosan/polyacrylic acid hydrogels. Int. J. Environ. Res. Public Health 2022, 19, 11123. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Di Martino, A.; Zednik, J.; Ozaltin, K.; Lovecka, L.; Bergerova, E.V.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Li, S.J.; Cai, M.J.; Wang, C.C.; Liu, Y.P. Ta3N5/CdS Core-shell S-scheme heterojunction nanofibers for efficient photocatalytic removal of antibiotic tetracycline and Cr(VI): Performance and mechanism insights. Adv. Fiber Mater. 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, T.; Zhang, Y.; Cai, J.F.; He, M.Q.; Li, M.Q.; Chen, Z.G.; Zhang, L. Growth of BiOBr/ZIF-67 nanocomposites on carbon fiber cloth as filter-membrane-shaped photocatalyst for degrading pollutants in flowing wastewater. Adv. Fiber Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Liu, X.G.; Zhang, Y.; Guo, X.T.; Pang, H. Electrospun metal-organic framework nanofiber membranes for energy storage and environmental protection. Adv. Fiber Mater. 2022, 4, 1463–1485. [Google Scholar] [CrossRef]
- Han, J.; Xing, W.Q.; Yan, J.; Wen, J.; Liu, Y.T.; Wang, Y.Q.; Wu, Z.F.; Tang, L.C.; Gao, J.F. Stretchable and superhydrophilic polyaniline/halloysite decorated nanofiber composite evaporator for high efficiency seawater desalination. Adv. Fiber Mater. 2022, 4, 1233–1245. [Google Scholar] [CrossRef]
- de Quadros Melo, D.; de Oliveira Sousa Neto, V.; de Freitas Barros, F.C.; Cabral Raulino, G.S.; Bastos Vidal, C.; do Nascimento, R.F. Chemical modifications of lignocellulosic materials and their application for removal of cations and anions from aqueous solutions. J. Appl. Polym. Sci. 2016, 133, 43286. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [PubMed]
- Bakar, N.A.; Othman, N.; Yunus, Z.M.; Hamood Altowayti, W.A.; Tahir, M.; Fitriani, N.; Mohd-Salleh, S.N.A. An insight review of lignocellulosic materials as activated carbon precursor for textile wastewater treatment. Environ. Technol. Innov. 2021, 22, 101445. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; AlSamman, M.; Oyarzun, D.; Del, C.; Pizarro, G.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.V.; Heleno, S.; Barros, L. Chemical composition and industrial applications of maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev. Environ. Sci. Biotechnol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent advances in eco-friendly composites derived from lignocellulosic biomass for wastewater treatment. Biomass. Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Nayak, A.K.; Naik, K.R.; Pal, A. Lignocellulosic-based sorbents. In Wastewater Treatment—Recycling, Management, and Valorization of Industrial Solid Wastes; Fahim, I.S., Said, L., Eds.; CRC Press: Boca Raton, FL, USA, 2023; Chapter 9; p. 74. [Google Scholar]
- Vasić, V.; Kukić, D.; Šćiban, M.; Đurišić-Mladenović, N.; Velić, N.; Pajin, B.; Crespo, J.; Farre, M.; Šereš, Z. Lignocellulose-based biosorbents for the removal of contaminants of emerging concern (CECs) from water: A review. Water 2023, 15, 1853. [Google Scholar] [CrossRef]
- Vázquez, G.; Antorrena, G.; González, J.; Doval, M.D. Adsorption of heavy metal ions by chemically modified Pinus pinaster bark. Bioresour. Technol. 1994, 48, 251–255. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Álvarez, J.; Freire, S.; Lόpez-Lorenzo, M.; Antorrena, G. Removal of cadmium and mercury ions from aqueous solution by sorption on treated Pinus pinaster bark: Kinetics and isotherms. Bioresour. Technol. 2002, 82, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Alonso, R.; Freire, S.; González-Álvarez, J.; Antorrena, G. Uptake of phenol from aqueous solutions by adsorption in a Pinus pinaster bark packed bed. J. Hazard. Mater. B 2006, 133, 61–67. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Álvarez, J.; García, A.I.; Freire, S.; Antorrena, G. Adsorption of phenol on formaldehyde-pretreated in a Pinus pinaster bark: Equilibrium and kinetics. Bioresour. Technol. 2007, 98, 1535–1540. [Google Scholar] [CrossRef]
- Brás, I.P.; Santos, L.; Alves, A. Organochlorine pesticides removal by pinus bark sorption. Environ. Sci. Technol. 1999, 33, 631–634. [Google Scholar] [CrossRef]
- Haussard, M.; Gaballah, I.; Donato, P.D.; Barrès, O.; Mourey, A. Removal of hydrocarbons from wastewater using treated bark. J. Air Waste Manag. Assoc. 2001, 51, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.C.; Cerrella, E.G.; Cukierman, A.L. Lignocellulosic materials as potential biosorbents of trace toxic metals from wastewater. Ind. Eng. Chem. Res. 2002, 41, 3580–3585. [Google Scholar] [CrossRef]
- Shin, E.W.; Karthikeyan, K.G.; Tshabalala, M.A. Adsorption mechanism of cadmium on juniper bark and wood. Bioresour. Technol. 2007, 98, 588–594. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Sousa, S.; Jiménez-Guerrero, P.; Ruiz, A.; Ratola, N.; Alves, A. Organochlorine pesticides removal from wastewater by pine bark adsorption after activated sludge treatment. Environ. Technol. 2011, 32, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Arim, A.L.; Cecílio, D.F.; Quina, M.J.; Gando-Ferreira, L.M. Development and characterization of pine bark with enhanced capacity for uptaking Cr (III) from aqueous solutions. Can. J. Chem. Eng. 2018, 96, 855–864. [Google Scholar] [CrossRef]
- Mongioví, C.; Crini, G. Copper recovery from aqueous solutions by hemp shives: Adsorption studies and modeling. Processes 2023, 11, 191. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherms models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Arellano, M.; Robledo-Ortíz, J.R. A critical overview of adsorption models linearization: Methodological and statistical inconsistencies. Sep. Purif. Rev. 2021, 51, 358–372. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar Band. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption of carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O.G. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Izvestiya Akademii Nauk. SSSR Otd. Khimicheskikh Nauk. 1962, 2, 209–216. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in lösungen. Z. Für Phys. Chem. 1906, 57, 385–471. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URSS 1940, 12, 217–256. [Google Scholar]
- Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.R.L.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from plant fibers of hemp and flax for metal removal: Comparison of their biosorption properties. Molecules 2021, 26, 4199. [Google Scholar] [CrossRef]












| Lagergren | Ho et McKay | Weber et Morris | Elovich | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ci | qe,exp | qe,cal | k1 | R2 | qe,cal | k2 | R2 | Kp | C | R2 | α | β | R2 | |
| Pin-W | 25 | 1.60 | 0.19 | 0.014 | 0.3087 | 1.59 | 0.303 | 0.9980 | 0.006 | 1.48 | 0.1448 | 6 × 1035 | 58 | 0.0632 |
| 50 | 2.06 | 0.28 | 0.018 | 0.3261 | 2.53 | 0.250 | 0.9996 | 0.013 | 2.33 | 0.3825 | 7 × 1022 | 24 | 0.2835 | |
| Pin-C | 25 | 2.53 | 0.69 | 0.020 | 0.7598 | 2.08 | 0.122 | 0.9997 | 0.052 | 1.38 | 0.6661 | 4 × 101 | 5 | 0.8622 |
| 50 | 4.26 | 0.101 | 0.020 | 0.6089 | 4.35 | 0.073 | 0.9994 | 0.058 | 3.47 | 0.6495 | 3 × 106 | 5 | 0.6049 | |
| Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qm | K | R2 | 1/nF | KF | R2 | B | KT | R2 | |
| Pin-W | 2.2 | 1.01 | 0.9813 | 0.26 | 0.70 | 0.8621 | 0.30 | 7.9 | 0.5659 |
| Pin-C | 3.9 | 0.10 | 0.8681 | 0.31 | 1.76 | 0.6002 | 0.79 | 27.9 | 0.4377 |
| Pin-R | Pin-W | Pin-C | |
|---|---|---|---|
| Klason lignin (%) | 30.7 | 25.4 | 26.5 |
| Lignin soluble (%) | 0.3 | 0.3 | 0.3 |
| Total lignin (%) | 31.0 | 25.8 | 26.7 |
| Extractive soluble water (%) | 4.8 | 4.4 | 0.9 |
| Acetone soluble extractives (%) | 1.7 | 1.6 | 0.1 |
| Total mining and quarrying (%) | 6.5 | 6.0 | 1.0 |
| Total cellulose (%) | 41.9 | 41.9 | 48.2 |
| Total hemicelluloses (%) | 20.0 | 18.6 | 21.1 |
| Ashes at 550 °C (%) | 0. | 0.4 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongioví, C.; Jaillet, M.; Lacalamita, D.; Morin-Crini, N.; Lecourt, M.; Tapin-Lingua, S.; Crini, G. A Strategy to Valorize a By-Product of Pine Wood (Pinus pinaster) for Copper Removal from Aqueous Solutions. Molecules 2023, 28, 6436. https://doi.org/10.3390/molecules28186436
Mongioví C, Jaillet M, Lacalamita D, Morin-Crini N, Lecourt M, Tapin-Lingua S, Crini G. A Strategy to Valorize a By-Product of Pine Wood (Pinus pinaster) for Copper Removal from Aqueous Solutions. Molecules. 2023; 28(18):6436. https://doi.org/10.3390/molecules28186436
Chicago/Turabian StyleMongioví, Chiara, Maélys Jaillet, Dario Lacalamita, Nadia Morin-Crini, Michael Lecourt, Sandra Tapin-Lingua, and Grégorio Crini. 2023. "A Strategy to Valorize a By-Product of Pine Wood (Pinus pinaster) for Copper Removal from Aqueous Solutions" Molecules 28, no. 18: 6436. https://doi.org/10.3390/molecules28186436
APA StyleMongioví, C., Jaillet, M., Lacalamita, D., Morin-Crini, N., Lecourt, M., Tapin-Lingua, S., & Crini, G. (2023). A Strategy to Valorize a By-Product of Pine Wood (Pinus pinaster) for Copper Removal from Aqueous Solutions. Molecules, 28(18), 6436. https://doi.org/10.3390/molecules28186436