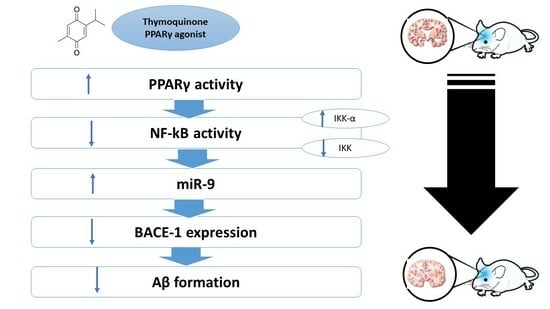
The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights
Abstract
:1. Introduction
2. Results
2.1. Behavioral Performance
2.2. Histopathological Examination
2.3. Immunohistochemical Examination
2.4. Effect on Mitochondrial Membrane Potential
2.5. Effect on Inflammatory Cytokines
2.6. Effect on IκB-α and IKK-α
2.7. Effect on miR-9, PPAR-γ, NF-κB, BACE-1, and Synapsin-2
2.8. Effect on Amyloid Beta
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Behavioral Tests
4.4. Sample Collection
4.5. Histopathological Examination
4.6. Immunohistochemical Examination
4.7. Determination of Mitochondrial Membrane Potential
4.8. Determination of Inflammatory Cytokines
4.9. Determination of IκB-α and IKK-α
4.10. Determination of PPAR-γ, NF-κB, BACE-1, Synapsin-2, and miR-9
4.11. Determination of Amyloid Beta
4.12. Determination of Phosphorylated Tau Protein (Ptau)
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Selkoe, D.J. Soluble Oligomers of the Amyloid β-Protein: Impair Synaptic Plasticity and Behavior. In Synaptic Plasticity and the Mechanism of Alzheimer’s Disease; Springer: Berlin/Heidelberg, Germany, 2008; Volume 192, pp. 89–102. [Google Scholar]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the Global Burden of Alzheimer’s Disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Mat Nuri, T.H.; Hong, Y.H.; Ming, L.C.; Mohd Joffry, S.; Othman, M.F.; Neoh, C.F. Knowledge on Alzheimer’s Disease among Public Hospitals and Health Clinics Pharmacists in the State of Selangor, Malaysia. Front. Pharmacol. 2017, 8, 739. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s Disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Singh, R.K. Recent Trends in the Management of Alzheimer’s Disease: Current Therapeutic Options and Drug Repurposing Approaches. Curr. Neuropharmacol. 2020, 18, 868–882. [Google Scholar] [CrossRef]
- Imran, M.; Al Kury, L.T.; Nadeem, H.; Shah, F.A.; Abbas, M.; Naz, S.; Khan, A.; Li, S. Benzimidazole Containing Acetamide Derivatives Attenuate Neuroinflammation and Oxidative Stress in Ethanol-Induced Neurodegeneration. Biomolecules 2020, 10, 108. [Google Scholar] [CrossRef]
- Rockwood, K. Biomarkers to Measure Treatment Effects in Alzheimer’s Disease: What Should We Look For? Int. J. Alzheimer’s Dis. 2011, 2011, 598175. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.; Bergman, J.; Vanderschuren, L.; Ellenbroek, B.; Willner, P. The Behavioural Pharmacology of Dementia. Behav. Pharmacol. 2017, 28, 91–93. [Google Scholar]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘Classical’ and ‘Non-Classical’ Functions and Pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of MicroRNA Cluster MiR-29a/b-1 in Sporadic Alzheimer’s Disease Correlates with Increased BACE1/β-Secretase Expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Morais, G.S., Jr.; Henriques, A.D.; Machado-Silva, W.; Perez, D.I.V.; Brito, C.J.; Camargos, E.F.; Moraes, C.F.; Nóbrega, O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients with Late-Onset Alzheimer Disease. Am. J. Alzheimer’s Dis. Other Dement. 2020, 35. [Google Scholar] [CrossRef] [PubMed]
- Schonrock, N.; Ke, Y.D.; Humphreys, D.; Staufenbiel, M.; Ittner, L.M.; Preiss, T.; Götz, J. Neuronal MicroRNA Deregulation in Response to Alzheimer’s Disease Amyloid-β. PLoS ONE 2010, 5, e11070. [Google Scholar] [CrossRef]
- Schonrock, N.; Humphreys, D.T.; Preiss, T.; Götz, J. Target Gene Repression Mediated by MiRNAs MiR-181c and MiR-9 Both of Which Are down-Regulated by Amyloid-β. J. Mol. Neurosci. 2012, 46, 324–335. [Google Scholar] [CrossRef]
- Che, H.; Sun, L.-H.; Guo, F.; Niu, H.-F.; Su, X.-L.; Bao, Y.-N.; Fu, Z.D.; Liu, H.; Hou, X.; Yang, B.-F. Expression of Amyloid-Associated MiRNAs in Both the Forebrain Cortex and Hippocampus of Middle-Aged Rat. Cell. Physiol. Biochem. 2014, 33, 11–22. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, L.; Xu, W.; Jing, P.; Zhan, P. MicroRNA-9 Attenuates Amyloidβ-induced Synaptotoxicity by Targeting Calcium/Calmodulin-Dependent Protein Kinase Kinase 2. Mol. Med. Rep. 2014, 9, 1917–1922. [Google Scholar] [CrossRef]
- Snow, W.M.; Albensi, B.C. Neuronal Gene Targets of NF-ΚB and Their Dysregulation in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef]
- Jones, S.V.; Kounatidis, I. Nuclear Factor-Kappa B and Alzheimer Disease, Unifying Genetic and Environmental Risk Factors from Cell to Humans. Front. Immunol. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Bronzuoli, M.R.; Iacomino, A.; Steardo, L.; Scuderi, C. Targeting Neuroinflammation in Alzheimer’s Disease. J. Inflamm. Res. 2016, 9, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome Proliferator-Activated Receptors (PPARs) as Therapeutic Target in Neurodegenerative Disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Gad, D.; Mansour, H.E.A.; Saad-Allah, K.M.; Abdallah, M.S.; Elberri, A.I.; Mosalam, E.M. Biostimulants Improve the Hepatoprotection of Ammi Visnaga Seed Yield Extract against Carbon Tetrachloride Induced Acute Hepatitis in Mice through Modulation of MAPK. Saudi J. Biol. Sci. 2022. [Google Scholar] [CrossRef]
- Abo Mansour, H.E.; El-Batsh, M.M.; Badawy, N.S.; Mehanna, E.T.; Mesbah, N.M.; Abo-Elmatty, D.M. Ginger Extract Loaded into Chitosan Nanoparticles Enhances Cytotoxicity and Reduces Cardiotoxicity of Doxorubicin in Hepatocellular Carcinoma in Mice. Nutr. Cancer 2021, 73, 2347–2362. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Abo Mansour, H.E. Ginger Extract Adjuvant to Doxorubicin in Mammary Carcinoma: Study of Some Molecular Mechanisms. Eur. J. Nutr. 2018, 57, 981–989. [Google Scholar] [CrossRef]
- Mosalam, E.M.; Zidan, A.-A.A.; Mehanna, E.T.; Mesbah, N.M.; Abo-Elmatty, D.M. Thymoquinone and Pentoxifylline Enhance the Chemotherapeutic Effect of Cisplatin by Targeting Notch Signaling Pathway in Mice. Life Sci. 2020, 244, 117299. [Google Scholar] [CrossRef]
- Zidan, A.-A.A.; El-Ashmawy, N.E.; Khedr, E.G.; Ebeid, E.-Z.M.; Salem, M.L.; Mosalam, E.M. Loading of Doxorubicin and Thymoquinone with F2 Gel Nanofibers Improves the Antitumor Activity and Ameliorates Doxorubicin-Associated Nephrotoxicity. Life Sci. 2018, 207, 461–470. [Google Scholar] [CrossRef]
- Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Thymoquinone Protects Cultured Rat Primary Neurons against Amyloid β-Induced Neurotoxicity. Biochem. Biophys. Res. Commun. 2013, 433, 362–367. [Google Scholar] [CrossRef]
- Bargi, R.; Asgharzadeh, F.; Beheshti, F.; Hosseini, M.; Sadeghnia, H.R.; Khazaei, M. The Effects of Thymoquinone on Hippocampal Cytokine Level, Brain Oxidative Stress Status and Memory Deficits Induced by Lipopolysaccharide in Rats. Cytokine 2017, 96, 173–184. [Google Scholar] [CrossRef]
- Beker, M.; Dallı, T.; Elibol, B. Thymoquinone Can Improve Neuronal Survival and Promote Neurogenesis in Rat Hippocampal Neurons. Mol. Nutr. Food Res. 2018, 62, 1700768. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Choi, B.J.; Chang, M.S.; Park, S.K. Nelumbo Nucifera Semen Extract Improves Memory in Rats with Scopolamine-Induced Amnesia through the Induction of Choline Acetyltransferase Expression. Neurosci. Lett. 2009, 461, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Chopin, P.; Briley, M. Effects of Four Non-Cholinergic Cognitive Enhancers in Comparison with Tacrine and Galanthamine on Scopolamine-Induced Amnesia in Rats. Psychopharmacology 1992, 106, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Nadeem, M.S.; Altayb, H.N. Sterubin Protects against Chemically-Induced Alzheimer’s Disease by Reducing Biomarkers of Inflammation-IL-6/IL-β/TNF-α and Oxidative Stress-SOD/MDA in Rats. Saudi J. Biol. Sci. 2023, 30, 103560. [Google Scholar] [CrossRef]
- AlGhamdi, S.A.; Al-Abbasi, F.A.; Alghamdi, A.M.; Omer, A.B.; Afzal, O.; Altamimi, A.S.A.; Alamri, A.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I. Barbigerone Prevents Scopolamine-Induced Memory Impairment in Rats by Inhibiting Oxidative Stress and Acetylcholinesterase Levels. R. Soc. Open Sci. 2023, 10, 230013. [Google Scholar] [CrossRef]
- Poorgholam, P.; Yaghmaei, P.; Hajebrahimi, Z. Thymoquinone Recovers Learning Function in a Rat Model of Alzheimer’s Disease. Avicenna J. Phytomed. 2018, 8, 188–197. [Google Scholar]
- He, Z.; Han, S.; Wu, C.; Liu, L.; Zhu, H.; Liu, A.; Lu, Q.; Huang, J.; Du, X.; Li, N. Bis (Ethylmaltolato) Oxidovanadium (Iv) Inhibited the Pathogenesis of Alzheimer’s Disease in Triple Transgenic Model Mice. Metallomics 2020, 12, 474–490. [Google Scholar] [CrossRef]
- Yuliana, A.; Daijo, A.; Jheng, H.-F.; Kwon, J.; Nomura, W.; Takahashi, H.; Ara, T.; Kawada, T.; Goto, T. Endoplasmic Reticulum Stress Impaired Uncoupling Protein 1 Expression via the Suppression of Peroxisome Proliferator-Activated Receptor γ Binding Activity in Mice Beige Adipocytes. Int. J. Mol. Sci. 2019, 20, 274. [Google Scholar] [CrossRef]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The Peroxisome Proliferator-Activated Receptor-γ Is a Negative Regulator of Macrophage Activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Majdalawieh, A.; Ro, H.-S. PPARγ1 and LXRα Face a New Regulator of Macrophage Cholesterol Homeostasis and Inflammatory Responsiveness, AEBP1. Nucl. Recept. Signal. 2010, 8, nrs-08004. [Google Scholar] [CrossRef]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by Pioglitazone in the MPTP Model of Parkinson’s Disease Correlates with IκBα Induction and Block of NFκB and INOS Activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Qian, Y.; Li, X.; Li, M. Pioglitazone Reduces β Amyloid Levels via Inhibition of PPARγ Phosphorylation in a Neuronal Model of Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 178. [Google Scholar] [CrossRef]
- Xiang, G.Q.; Tang, S.S.; Jiang, L.Y.; Hong, H.; Li, Q.; Wang, C.; Wang, X.Y.; Zhang, T.T.; Yin, L. PPAR γ Agonist Pioglitazone Improves Scopolamine-Induced Memory Impairment in Mice. J. Pharm. Pharmacol. 2012, 64, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.; Gallucci, G.; Tong, M.; de la Monte, S.M. Therapeutic Advantages of Dual Targeting of PPAR-δ and PPAR-γ in an Experimental Model of Sporadic Alzheimer’s Disease. J. Park. Dis. Alzheimer’s Dis. 2018, 5. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Zhao, H. Thymoquinone Reduces Spinal Cord Injury by Inhibiting Inflammatory Response, Oxidative Stress and Apoptosis via PPAR-γ and PI3K/Akt Pathways. Exp. Ther. Med. 2018, 15, 4987–4994. [Google Scholar] [CrossRef]
- Hussien, N.I.; Elawady, M.A.; Elmaghrabi, M.M.; Muhammad, M.H. Impact of Thymoquinone on Memory Deficit-Associated with Global Cerebral Ischemia-Reperfusion Injury in Rats; Possible Role of PPAR-γ. Am. J. Biomed. Sci. 2020, 12, 77–90. [Google Scholar] [CrossRef]
- Zaher, M.; Bendary, M.; El-Aziz, G.; Ali, A. Potential Protective Role of Thymoquinone on Experimentally-induced Alzheimer Rats. J. Pharm. Res. Int. 2019, 31, 1–18. [Google Scholar] [CrossRef]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory Feedback Control of NF-ΚB Signalling in Health and Disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Bauer, D.; Soliman, K.F.A. The Antioxidant Effects of Thymoquinone in Activated BV-2 Murine Microglial Cells. Neurochem. Res. 2016, 41, 3227–3238. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone Alleviates the Experimentally Induced Alzheimer’s Disease Inflammation by Modulation of TLRs Signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Thummuri, D.; Jeengar, M.K.; Shrivastava, S.; Nemani, H.; Ramavat, R.N.; Chaudhari, P.; Naidu, V.G.M. Thymoquinone Prevents RANKL-Induced Osteoclastogenesis Activation and Osteolysis in an in Vivo Model of Inflammation by Suppressing NF-KB and MAPK Signalling. Pharmacol. Res. 2015, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mosalam, E.M.; Elberri, A.I.; Sallam, A.S.; Salem, H.R.; Metwally, E.M.; Abdallah, M.S.; Shaldam, M.A.; Mansour, H.E.A. Chronotherapeutic Neuroprotective Effect of Verapamil against Lipopolysaccharide-Induced Neuroinflammation in Mice through Modulation of Calcium-Dependent Genes. Mol. Med. 2022, 28, 139. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Schmitt, K.; Götz, J. Mitochondrial Dysfunction-the Beginning of the End in Alzheimer’s Disease? Separate and Synergistic Modes of Tau and Amyloid-β Toxicity. Alzheimer’s Res. Ther. 2011, 3, 15. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of Acidic Oligosaccharide Sugar Chain on Scopolamine-Induced Memory Impairment in Rats and Its Related Mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef]
- El-Sherbiny, D.A.; Khalifa, A.E.; Attia, A.S.; Eldenshary, E.E.-D.S. Hypericum Perforatum Extract Demonstrates Antioxidant Properties against Elevated Rat Brain Oxidative Status Induced by Amnestic Dose of Scopolamine. Pharmacol. Biochem. Behav. 2003, 76, 525–533. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-Enhancing and Antioxidant Activities of Iridoid Glycosides from Scrophularia Buergeriana in Scopolamine-Treated Mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Ismail, N.; Ismail, M.; Mazlan, M.; Latiff, L.A.; Imam, M.U.; Iqbal, S.; Azmi, N.H.; Ghafar, S.A.A.; Chan, K.W. Thymoquinone Prevents β-Amyloid Neurotoxicity in Primary Cultured Cerebellar Granule Neurons. Cell. Mol. Neurobiol. 2013, 33, 1159–1169. [Google Scholar] [CrossRef]
- Qian, D.; Wei, G.; Xu, C.; He, Z.; Hua, J.; Li, J.; Hu, Q.; Lin, S.; Gong, J.; Meng, H. Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Repair Acute Necrotized Pancreatitis by Secreting MicroRNA-9 to Target the NF-ΚB1/P50 Gene in Rats. Sci. Rep. 2017, 7, 581. [Google Scholar] [CrossRef]
- Lee, W.S.; Yasuda, S.; Kono, M.; Kudo, Y.; Shimamura, S.; Kono, M.; Fujieda, Y.; Kato, M.; Oku, K.; Shimizu, T. MicroRNA-9 Ameliorates Destructive Arthritis through down-Regulation of NF-ΚB1-RANKL Pathway in Fibroblast-like Synoviocytes. Clin. Immunol. 2020, 212, 108348. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Liu, N.; Luo, S.; Huang, W.; Zha, Z.; Yang, J. MicroRNA-9 Regulates the Development of Knee Osteoarthritis through the NF-KappaB1 Pathway in Chondrocytes. Medicine 2016, 95, e4315. [Google Scholar] [CrossRef] [PubMed]
- Giusti, S.A.; Vogl, A.M.; Brockmann, M.M.; Vercelli, C.A.; Rein, M.L.; Trümbach, D.; Wurst, W.; Cazalla, D.; Stein, V.; Deussing, J.M. MicroRNA-9 Controls Dendritic Development by Targeting REST. eLife 2014, 3, e02755. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M. MiR-9 Promotes Microglial Activation by Targeting MCPIP1. Nat. Commun. 2014, 5, 4386. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Gao, P.; Wang, L.-T.; Yan, Y.-H.; Xia, Y.; Song, J.; Li, H.-Y.; Yang, J.-X. Osthole Stimulated Neural Stem Cells Differentiation into Neurons in an Alzheimer’s Disease Cell Model via Upregulation of MicroRNA-9 and Rescued the Functional Impairment of Hippocampal Neurons in APP/PS1 Transgenic Mice. Front. Neurosci. 2017, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. The Functional Role of MicroRNAs in the Pathogenesis of Tauopathy. Cells 2020, 9, 2262. [Google Scholar] [CrossRef]
- Hitt, B.; Riordan, S.M.; Kukreja, L.; Eimer, W.A.; Rajapaksha, T.W.; Vassar, R. β-Site Amyloid Precursor Protein (APP)-Cleaving Enzyme 1 (BACE1)-Deficient Mice Exhibit a Close Homolog of L1 (CHL1) Loss-of-Function Phenotype Involving Axon Guidance Defects. J. Biol. Chem. 2012, 287, 38408–38425. [Google Scholar] [CrossRef]
- Peters, F.; Salihoglu, H.; Rodrigues, E.; Herzog, E.; Blume, T.; Filser, S.; Dorostkar, M.; Shimshek, D.R.; Brose, N.; Neumann, U. BACE1 Inhibition More Effectively Suppresses Initiation than Progression of β-Amyloid Pathology. Acta Neuropathol. 2018, 135, 695–710. [Google Scholar] [CrossRef]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re Reduces Aβ Production by Activating PPARγ to Inhibit BACE1 in N2a/APP695 Cells. Eur. J. Pharmacol. 2016, 793, 101–108. [Google Scholar] [CrossRef]
- Lin, N.; Chen, L.; Pan, X.; Zhu, Y.; Zhang, J.; Shi, Y.; Chen, X. Tripchlorolide Attenuates β-Amyloid Generation via Suppressing PPARγ-Regulated BACE1 Activity in N2a/APP695 Cells. Mol. Neurobiol. 2016, 53, 6397–6406. [Google Scholar] [CrossRef]
- Jahan, R.; Yousaf, M.; Khan, H.; Shah, S.A.; Khan, A.A.; Bibi, N.; Javed, F.; Ijaz, M.; Ali, A.; Wei, D.-Q. Zinc Ortho Methyl Carbonodithioate Improved Pre and Post-Synapse Memory Impairment via SIRT1/p-JNK Pathway against Scopolamine in Adult Mice. J. Neuroimmune Pharmacol. 2023, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone Administration Ameliorates Alzheimer’s Disease-like Phenotype by Promoting Cell Survival in the Hippocampus of Amyloid Beta1–42 Infused Rat Model. Phytomedicine 2020, 79, 153324. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-Rich Fraction Nanoemulsion (TQRFNE) Decreases Aβ40 and Aβ42 Levels by Modulating APP Processing, up-Regulating IDE and LRP1, and down-Regulating BACE1 and RAGE in Response to High Fat/Cholesterol Diet-Induced Rats. Biomed. Pharmacother. 2017, 95, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Corradi, A.; Zanardi, A.; Giacomini, C.; Onofri, F.; Valtorta, F.; Zoli, M.; Benfenati, F. Synapsin-I-and Synapsin-II-Null Mice Display an Increased Age-Dependent Cognitive Impairment. J. Cell Sci. 2008, 121, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J. NF-κB-Regulated Micro RNAs (MiRNAs) in Primary Human Brain Cells. Exp. Neurol. 2012, 235, 484–490. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Cheng, Y.-C.; Chen, H.-M.; Liang, Y.-J.; Yen, C.-H. Rosiglitazone Promotes Neurite Outgrowth and Mitochondrial Function in N2A Cells via PPARgamma Pathway. Mitochondrion 2014, 14, 7–17. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, D.; Yu, H.; Luo, X.; Wu, W. Ginkgo Biloba Extract Ameliorates Scopolamine-Induced Memory Deficits via Rescuing Synaptic Damage. Curr. Med. Sci. 2022, 42, 474–482. [Google Scholar] [CrossRef]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 Ameliorates Behavioral Abnormalities and Modulates the Hippocampal Proteomic Change in Triple Transgenic Mice of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Loganin Improves Learning and Memory Impairments Induced by Scopolamine in Mice. Eur. J. Pharmacol. 2009, 619, 44–49. [Google Scholar] [CrossRef]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor Effect of Thymoquinone Combined with Resveratrol on Mice Transplanted with Breast Cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]









| Normal Control | SCOP | SCOP + TQ | |
|---|---|---|---|
| TNF-α (pg/mL) | 29.857 ± 3.913 | 154.928 ± 12.789 a | 94.517 ± 5.287 a,b |
| IL-6 (pg/mL) | 27.8 ± 6.142 | 77 ± 5.597 a | 51 ± 5.83 a,b |
| Gene | Forward | Reverse |
|---|---|---|
| PPAR-γ | ATCAGGTTTGGGCGGGATCG | GTCAAGATCGCCCTCGCCTT |
| NF-κB | TCTCGACCTCCACCGGATCT | GCCCGCCTAAGGTGAAGAGA |
| BACE-1 | GGGAGCTGGGAGCTGGATTA | CCCAGCTACATCTGGACGC |
| Synapsin-2 | GTGTGGTCTTCCAGGACCTGAT | GCTGTCGGATGAGCACAAAGTC |
| β-actin | GCTCCTCCTGAGCGCAAGTA | GCAGCTCAGTAACAGTCCGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo Mansour, H.E.; Elberri, A.I.; Ghoneim, M.E.-S.; Samman, W.A.; Alhaddad, A.A.; Abdallah, M.S.; El-Berri, E.I.; Salem, M.A.; Mosalam, E.M. The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights. Molecules 2023, 28, 6566. https://doi.org/10.3390/molecules28186566
Abo Mansour HE, Elberri AI, Ghoneim ME-S, Samman WA, Alhaddad AA, Abdallah MS, El-Berri EI, Salem MA, Mosalam EM. The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights. Molecules. 2023; 28(18):6566. https://doi.org/10.3390/molecules28186566
Chicago/Turabian StyleAbo Mansour, Hend E., Aya Ibrahim Elberri, Mai El-Sayed Ghoneim, Waad A. Samman, Aisha A. Alhaddad, Mahmoud S. Abdallah, Eman I. El-Berri, Mohamed A. Salem, and Esraa M. Mosalam. 2023. "The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights" Molecules 28, no. 18: 6566. https://doi.org/10.3390/molecules28186566
APA StyleAbo Mansour, H. E., Elberri, A. I., Ghoneim, M. E. -S., Samman, W. A., Alhaddad, A. A., Abdallah, M. S., El-Berri, E. I., Salem, M. A., & Mosalam, E. M. (2023). The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights. Molecules, 28(18), 6566. https://doi.org/10.3390/molecules28186566