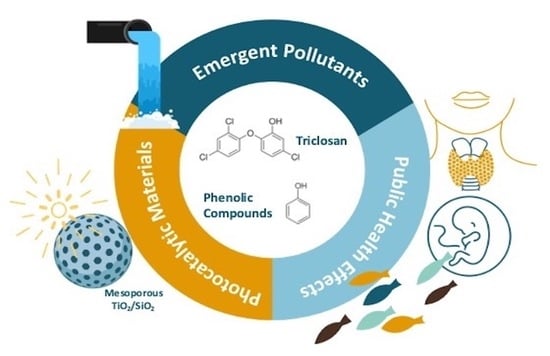
Nanomaterials for Removal of Phenolic Derivatives from Water Systems: Progress and Future Outlooks
Abstract
:1. Introduction
2. Nanomaterials for Adsorption of TCS and Phenolic Derivativess
3. Degradation of TCS and Related Phenolic Compounds
3.1. Formation of Carcinogenic Intermediates
3.2. Effect of Solution pH
3.3. Effects of Competing Species on the Degradation of TCS and Related Compounds
4. Photocatalysis
4.1. Mesoporous Hybrid Materials
4.2. Functionalized Nanoporous Hybrid Materials
4.3. Mesoporous Metals and Metal Oxide-Supported Metals for Photodegradation of TCS and Other Phenolic Compounds and Their Derivatives
4.4. Multifunctional Photocatalysts
5. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasse, C.; Ford, B.; Nomura, D.K.; Sedlak, D.L. Unexpected Transformation of Dissolved Phenols to Toxic Dicarbonyls by Hydroxyl Radicals and UV Light. Proc. Natl. Acad. Sci. USA 2018, 115, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.D.; Jampani, H.B.; Newman, J.L.; Lee, A.S. Triclosan: A Review of Effectiveness and Safety in Health Care Settings. Am. J. Infect. Control. 2000, 28, 184–196. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan Targets Lipid Synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Iwata, H. Risk Assessment of Triclosan in the Global Environment Using a Probabilistic Approach. Ecotoxicol. Environ. Saf. 2017, 143, 111–119. [Google Scholar] [CrossRef]
- Constantin, L.A.; Nitoi, I.; Cristea, N.I.; Constantin, M.A. Possible Degradation Pathways of Triclosan from Aqueous Systems via TiO2 Assisted Photocatalyis. J. Ind. Eng. Chem. 2018, 58, 155–162. [Google Scholar] [CrossRef]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive Insight into Triclosan—From Widespread Occurrence to Health Outcomes. Environ. Sci. Pollut. Res. 2021, 1, 1–22. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Lee, D.G.; Zhao, F.; Rezenom, Y.H.; Russell, D.H.; Chu, K.H. Biodegradation of Triclosan by a Wastewater Microorganism. Water Res. 2012, 46, 4226–4234. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, P.; Zhang, Z.; Shi, J.; Jiao, Z.; Shao, B. Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PLoS ONE 2016, 11, e0154758. [Google Scholar] [CrossRef]
- Dar, O.I.; Aslam, R.; Pan, D.; Sharma, S.; Andotra, M.; Kaur, A.; Jia, A.Q.; Faggio, C. Source, Bioaccumulation, Degradability and Toxicity of Triclosan in Aquatic Environments: A Review. Environ. Technol. Innov. 2022, 25, 102122. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Bester, K. Degradation of Triclosan by Environmental Microbial Consortia and by Axenic Cultures of Microorganisms with Concerns to Wastewater Treatment. Appl. Microbiol. Biotechnol. 2018, 102, 5403–5417. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence Statement on Triclosan and Triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.J.; Kim, H.Y.; Kim, M.K.; Seo, D.W.; Lee, B.M.; Kim, K.B. Risk Assessment of Triclosan, a Cosmetic Preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, HHS. Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final rule. Fed. Regist. 2017, 82, 60474–60503. Available online: https://pubmed.ncbi.nlm.nih.gov/29260839/ (accessed on 25 August 2023).
- González-Pérez, B.K.; Sarma, S.S.S.; Castellanos-Páez, M.E.; Nandini, S. Multigenerational Effects of Triclosan on the Demography of Plationus Patulus and Brachionus Havanaensis (ROTIFERA). Ecotoxicol. Environ. Saf. 2018, 147, 275–282. [Google Scholar] [CrossRef]
- Bakare, B.F.; Adeyinka, G.C. Occurrence and Fate of Triclosan and Triclocarban in Selected Wastewater Systems across Durban Metropolis, KwaZulu-Natal, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 6769. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Triclosan: A Widespread Environmental Toxicant with Many Biological Effects|Annual Review of Pharmacology and Toxicology. Available online: https://www.annualreviews.org/doi/10.1146/annurev-pharmtox-010715-103417 (accessed on 25 August 2023).
- European Commission. Directorate General for Health and Consumers. Opinion on Triclosan: COLIPA N° P27. Addendum to the SCCP Opinion on Triclosan (SCCP/1192/08) from January 2009; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- COVID-19 Impact and Recovery Analysis—Phenol Market 2020–2024|Increasing Demand for Phenol from Developing Economies to Boost Growth|Technavio. Available online: https://www.businesswire.com/news/home/20200703005198/en/COVID-19-Impact-and-Recovery-Analysis---Phenol-Market-2020-2024-Increasing-Demand-for-Phenol-from-Developing-Economies-to-Boost-Growth-Technavio (accessed on 25 August 2023).
- Olaniyan, L.W.B.; Mkwetshana, N.; Okoh, A.I. Triclosan in Water, Implications for Human and Environmental Health. Springerplus 2016, 5, 1639. [Google Scholar] [CrossRef]
- Gorenoglu, E.; Aydin, E.; Topuz, E.; Pehlivanoglu-Mantas, E. Effect of Triclosan and Its Photolysis Products on Marine Bacterium V. fischeri and Freshwater Alga R. subcapitata. J. Environ. Manag. 2018, 211, 218–224. [Google Scholar] [CrossRef]
- Tatarazako, N.; Ishibashi, H.; Teshima, K.; Kishi, K.; Arizono, K. Effects of Triclosan on Various Aquatic Organisms. Environ. Sci. 2004, 11, 133–140. [Google Scholar]
- Butler, E.; Whelan, M.J.; Ritz, K.; Sakrabani, R.; van Egmond, R. The Effect of Triclosan on Microbial Community Structure in Three Soils. Chemosphere 2012, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.S.; Peng, F.-J.; Ying, G.G.; van den Brink, P.J. Fate and Effects of Triclosan in Subtropical River Biofilms. Aquat. Toxicol. 2019, 212, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.H.; Hsiao, C.D.; Kawakami, K.; Tse, W.K.F. Triclosan (TCS) Exposure Impairs Lipid Metabolism in Zebrafish Embryos. Aquat. Toxicol. 2016, 173, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, L.M.; Gibson, E.K.; Jeffay, S.C.; Crofton, K.M.; Setzer, W.R.; Cooper, R.L.; Stoker, T.E. The Effects of Triclosan on Puberty and Thyroid Hormones in Male Wistar Rats. Toxicol. Sci. 2009, 107, 56–64. [Google Scholar] [CrossRef]
- Frontiers|Triclosan Impairs Hippocampal Synaptic Plasticity and Spatial Memory in Male Rats. Available online: https://www.frontiersin.org/articles/10.3389/fnmol.2018.00429/full (accessed on 26 August 2023).
- Ajao, C.; Andersson, M.A.; Teplova, V.V.; Nagy, S.; Gahmberg, C.G.; Andersson, L.C.; Hautaniemi, M.; Kakasi, B.; Roivainen, M.; Salkinoja-Salonen, M. Mitochondrial Toxicity of Triclosan on Mammalian Cells. Toxicol. Rep. 2015, 2, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-N.-S.; Nguyen, T.-T.; Pham, T.-H.-T.; Ngo, M.-T.; Le, M.-V. Photocatalytic Degradation of Phenol in Aqueous Solutions Using TiO2/SiO2 Composite. Chem. Eng. Trans. 2020, 78, 427–432. [Google Scholar] [CrossRef]
- Maharana, D.; Niu, J.; Rao, N.N.; Xu, Z.; Shi, J. Electrochemical Degradation of Triclosan at a Ti/SnO2-Sb/Ce-PbO2 Anode. Clean Soil Air Water 2015, 43, 958–966. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, J.; Pan, H.; Hao, F.; Yao, J. Electrochemical Oxidation of Triclosan Using Ti/TiO2 NTs/Al–PbO2 Electrode: Reaction Mechanism and Toxicity Evaluation. Environ. Sci. Pollut. Res. 2021, 28, 26479–26487. [Google Scholar] [CrossRef]
- Song, Z.; Wang, N.; Zhu, L.; Huang, A.; Zhao, X.; Tang, H. Efficient Oxidative Degradation of Triclosan by Using an Enhanced Fenton-like Process. Chem. Eng. J. 2012, 198–199, 379–387. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Y.; Zhang, C.; Miao, D.; Li, J.; Liu, H.; Wang, L.; Gao, S. Removal of Triclosan in a Fenton-like System Mediated by Graphene Oxide: Reaction Kinetics and Ecotoxicity Evaluation. Sci. Total Environ. 2019, 673, 726–733. [Google Scholar] [CrossRef]
- Behera, S.K.; Oh, S.Y.; Park, H.S. Sorption of Triclosan onto Activated Carbon, Kaolinite and Montmorillonite: Effects of PH, Ionic Strength, and Humic Acid. J. Hazard. Mater. 2010, 179, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Khori, N.K.E.M.; Hadibarata, T.; Elshikh, M.S.; Al-Ghamdi, A.A.; Salmiati; Yusop, Z. Triclosan Removal by Adsorption Using Activated Carbon Derived from Waste Biomass: Isotherms and Kinetic Studies. J. Chin. Chem. Soc. 2018, 65, 951–959. [Google Scholar] [CrossRef]
- Shih, H.K.; Lin, C.W.; Ponnusamy, V.K.; Ramkumar, A.; Jen, J.F. Rapid Analysis of Triclosan in Water Samples Using an In-Tube Ultrasonication Assisted Emulsification Microextraction Coupled with Gas Chromatography-Electron Capture Detection. Anal. Methods 2013, 5, 2352–2359. [Google Scholar] [CrossRef]
- Yu, J.C.; Kwong, T.Y.; Luo, Q.; Cai, Z. Photocatalytic Oxidation of Triclosan. Chemosphere 2006, 65, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; San-Román, M.-F.; Ortiz, I. Photocatalytic Transformation of Triclosan. Reaction Products and Kinetics. Catalysts 2020, 10, 1468. [Google Scholar] [CrossRef]
- Solá-Gutiérrez, C.; Schröder, S.; San-Román, M.F.; Ortiz, I. Critical Review on the Mechanistic Photolytic and Photocatalytic Degradation of Triclosan. J. Environ. Manag. 2020, 260, 110101. [Google Scholar] [CrossRef]
- Munoz, M.; Pedro, Z.M.D.; Casas, J.A.; Rodriguez, J.J. Triclosan Breakdown by Fenton-like Oxidation. Chem. Eng. J. 2012, 198–199, 275–281. [Google Scholar] [CrossRef]
- Peng, J.; Shi, H.; Li, J.; Wang, L.; Wang, Z.; Gao, S. Bicarbonate Enhanced Removal of Triclosan by Copper(II) Catalyzed Fenton-like Reaction in Aqueous Solution. Chem. Eng. J. 2016, 306, 484–491. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, M.; Liu, J.; Ye, J.; Asefa, T. Facile Synthesis of an Effective G-C3N4-Based Catalyst for Advanced Oxidation Processes and Degradation of Organic Compounds. J. Mater. Chem. A 2021, 9, 14841–14850. [Google Scholar] [CrossRef]
- Jayalatha, N.A.; Devatha, C.P. Degradation of Triclosan from Domestic Wastewater by Biosurfactant Produced from Bacillus Licheniformis. Mol. Biotechnol. 2019, 61, 674–680. [Google Scholar] [CrossRef]
- Taştan, B.E.; Dönmez, G. Biodegradation of Pesticide Triclosan by A.Versicolor in Simulated Wastewater and Semi-Synthetic Media. Pestic. Biochem. Physiol. 2015, 118, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, K.; Vargas-Valentín, D.A.; Hernández-Maldonado, A.J. Adsorption of Contaminants of Emerging Concern from Aqueous Solutions Using Cu2+ Amino Grafted SBA-15 Mesoporous Silica: Multicomponent and Metabolites Adsorption. Ind. Eng. Chem. Res. 2018, 57, 6426–6439. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green Adsorbents for Wastewaters: A Critical Review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef]
- Anum, A.; Nazir, M.A.; Ibrahim, S.M.; Shah, S.S.A.; Tahir, A.A.; Malik, M.; Wattoo, M.A.; ur Rehman, A. Synthesis of Bi-Metallic-Sulphides/MOF-5@graphene Oxide Nanocomposites for the Removal of Hazardous Moxifloxacin. Catalysts 2023, 13, 984. [Google Scholar] [CrossRef]
- Margaret, S.M.; Paul Winston, A.J.P.; Muthupandi, S.; Shobha, P.; Sagayaraj, P. Enhanced Photocatalytic Degradation of Phenol Using Urchin-Like ZnO Microrod-Reduced Graphene Oxide Composite under Visible-Light Irradiation. J. Nanomater. 2021, 2021, e5551148. [Google Scholar] [CrossRef]
- Marcos-Hernández, M.; Villagrán, D. Mesoporous Composite Nanomaterials for Dye Removal and Other Applications. In Composites and Nanoadsorbents: Micro and Nano Technologies; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Elsevier: Amstrdam, The Netherlands, 2019; Chapter 11; pp. 265–293. [Google Scholar] [CrossRef]
- Wilson, B.E.; Rudisill, S.G.; Stein, A. Use of a Sacrificial Layer for an Efficient EISA Synthesis of Mesoporous Carbon. Microporous Mesoporous Mater. 2014, 197, 174–179. [Google Scholar] [CrossRef]
- Yokoyama, J.T.C.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Ronix, A.; Silva, M.C.; Silva, T.L.; Almeida, V.C. Stevia Residue as New Precursor of CO2-Activated Carbon: Optimization of Preparation Condition and Adsorption Study of Triclosan. Ecotoxicol. Environ. Saf. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Huang, S.C.; Juang, R.S. Characteristics of Pseudo-Second-Order Kinetic Model for Liquid-Phase Adsorption: A Mini-Review. Chem. Eng. J. 2009, 151, 1–9. [Google Scholar] [CrossRef]
- Simonin, J.P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Prisciandaro, M.; Musmarra, D. Triclosan Photolysis: Operating Condition Study and Photo-Oxidation Pathway. Chem. Eng. J. 2019, 377, 121045. [Google Scholar] [CrossRef]
- Catalysts|Free Full-Text|Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study. Available online: https://www.mdpi.com/2073-4344/9/8/640 (accessed on 26 August 2023).
- Gholizadeh, F.; Dianat, M.J.; Izadbakhsh, A. Photocatalytic Degradation of Phenol Using Silica SBA-16 Supported TiO2. Reac. Kinet. Mech. Cat. 2020, 130, 1171–1192. [Google Scholar] [CrossRef]
- Anawar, H.M.; Ahmed, G. Chapter 10—Combined Electrochemical-Advanced Oxidation and Enzymatic Process for Treatment of Wastewater Containing Emerging Organic Contaminants. In Emerging and Nanomaterial Contaminants in Wastewater; Mishra, A.K., Anawar, H.M.D., Drouiche, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–307. [Google Scholar] [CrossRef]
- Czech, B.; Kończak, M.; Rakowska, M.; Oleszczuk, P. Engineered Biochars from Organic Wastes for the Adsorption of Diclofenac, Naproxen and Triclosan from Water Systems. J. Clean. Prod. 2021, 288, 125686. [Google Scholar] [CrossRef]
- Aranami, K.; Readman, J.W. Photolytic Degradation of Triclosan in Freshwater and Seawater. Chemosphere 2007, 66, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Fabbri, D.; Minella, M.; Brigante, M.; Mailhot, G.; Maurino, V.; Minero, C.; Vione, D. New Insights into the Environmental Photochemistry of 5-Chloro-2-(2,4-Dichlorophenoxy)Phenol (Triclosan): Reconsidering the Importance of Indirect Photoreactions. Water Res. 2015, 72, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, J.; Chen, W.; Ma, X.; Li, G.; Chen, G.; Deng, J. Degradation of Triclosan by Chlorine Dioxide: Reaction Mechanism,2,4-Dichlorophenol Accumulation and Toxicity Evaluation. Chemosphere 2018, 207, 449–456. [Google Scholar] [CrossRef]
- Marazuela, M.D.; García-Fresnadillo, D. An Integrated Photosensitizing/Adsorbent Material for the Removal of Triclosan from Water Samples. Sep. Purif. Technol. 2020, 251, 117392. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic PH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Hartmann, P.; Leiner, M.J.P.; Kohlbacher, P. Photobleaching of a Ruthenium Complex in Polymers Used for Oxygen Optodes and Its Inhibition by Singlet Oxygen Quenchers. Sens. Actuators B Chem. 1998, 51, 196–202. [Google Scholar] [CrossRef]
- Zhang, T.; Low, J.; Yu, J.; Tyryshkin, A.M.; Mikmeková, E.; Asefa, T. A Blinking Mesoporous TiO2-x Composed of Nanosized Anatase with Unusually Long-Lived Trapped Charge Carriers. Angew. Chem. Int. Ed. Engl. 2020, 59, 15000–15007. [Google Scholar] [CrossRef]
- Kar, P.; Aggarwal, D.; Shukla, K.; Gupta, R.K. Defect State Modulation of TiO2 Nanostructures for Photocatalytic Abatement of Emerging Pharmaceutical Pollutant in Wastewater Effluent. Adv. Energy Sustain. Res. 2022, 3, 2100162. [Google Scholar] [CrossRef]
- Son, H.-S.; Ko, G.; Zoh, K.-D. Kinetics and Mechanism of Photolysis and TiO2 Photocatalysis of Triclosan. J. Hazard. Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Belekbir, S.; El Azzouzi, M.; Hamidi, A.E.; Rodríguez-Lorenzo, L.; Santaballa, J.A.; Canle, M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials 2020, 10, E996. [Google Scholar] [CrossRef] [PubMed]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid Materials for Heterogeneous Photocatalytic Degradation of Antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid Materials for the Removal of Emerging Pollutants in Water: Classification, Synthesis, and Properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Babu, P.; Naik, B. Cu-Ag Bimetal Alloy Decorated SiO2@TiO2 Hybrid Photocatalyst for Enhanced H2 Evolution and Phenol Oxidation under Visible Light. Inorg. Chem. 2020, 59, 10824–10834. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; ur Rehman, A. Engineering of a Hybrid G-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Tahir, A.A.; Nazir, M.A.; Shah, S.S.A.; Wattoo, M.A.; Kousar, R.; ur Rehman, A. Novel Approach towards Ternary Magnetic G-C3N4/ZnO-W/Snx Nanocomposite: Photodegradation of Nicotine under Visible Light Irradiation. Int. J. Environ. Anal. Chem. 2023, 1–19. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Qu, Y. TiO2-Based Photocatalytic Process for Purification of Polluted Water: Bridging Fundamentals to Applications. J. Nanomater. 2013, 2013, 319637. [Google Scholar] [CrossRef]
- Hosseini-Hosseinabad, S.M.; Moakhar, R.S.; Soleimani, F.; Sadrnezhaad, S.K.; Masudy-Panah, S.; Katal, R.; Seza, A.; Ghane, N.; Ramakrishna, S. One-Pot Microwave Synthesis of Hierarchical C-Doped CuO Dandelions/g-C3N4 Nanocomposite with Enhanced Photostability for Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2020, 530, 147271. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Antibiofilm Activity and Bioactive Phenolic Compounds of Ethanol Extract from the Hericium Erinaceus Basidiome. J. Adv. Pharm. Technol. Res. 2022, 13, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Badawy, A.A. Characterization and Use for the Degradation of Phenol, of New Core-Shell Mesoporous Si–Sn–Zr Oxides Synthesized by a Surfactant-Assisted Sol–Gel Self-Combustion Method. J. Sol-Gel Sci. Technol. 2022, 101, 453–467. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Nasser, W.S.; Osman, T.A.; Knebel, A.; Sánchez, E.P.V.; Hashem, T. Rapid Photocatalytic Degradation of Phenol from Water Using Composite Nanofibers under UV. Environ. Sci. Eur. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Brigante, M.; Schulz, P.C. Remotion of the Antibiotic Tetracycline by Titania and Titania–Silica Composed Materials. J. Hazard. Mater. 2011, 192, 1597–1608. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Wang, P. Rational Design of Nanomaterials for Water Treatment. Nanoscale 2015, 7, 17167–17194. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Shi, Y.; Zhang, Y. Structure-Dependent Adsorptive or Photocatalytic Performances of Solid and Hollow Dendritic Mesoporous Silica & Titania Nanospheres. Microporous Mesoporous Mater. 2020, 305, 110326. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized Mesoporous SBA-15 Silica: Recent Trends and Catalytic Applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Toufaily, J.; Koubaissy, B.; Kafrouny, L.; Hamad, H.; Magnoux, P.; Ghannam, L.; Karout, A.; Hazimeh, H.; Nemra, G.; Hamieh, M.; et al. Functionalization of SBA-15 Materials for the Adsorption of Phenols from Aqueous Solution. Open Eng. 2013, 3, 126–134. [Google Scholar] [CrossRef]
- Li, W.; Jia, X.; Li, P.; Zhang, B.; Zhang, H.; Geng, W.; Zhang, Q. Hollow Mesoporous SiO2–BiOBr Nanophotocatalyst: Synthesis, Characterization and Application in Photodegradation of Organic Dyes under Visible-Light Irradiation. ACS Sustain. Chem. Eng. 2015, 3, 1101–1110. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic–Organic Mesoporous Silicates—Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Wang, H.; Kang, J.; Liu, H.; Qu, J. Preparation of Organically Functionalized Silica Gel as Adsorbent for Copper Ion Adsorption. J. Environ. Sci. 2009, 21, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Sahoo, D.P.; Parida, K.M. Bimetallic Co-Effect of Au-Pd Alloyed Nanoparticles on Mesoporous Silica Modified g-C3N4 for Single and Simultaneous Photocatalytic Oxidation of Phenol and Reduction of Hexavalent Chromium. J. Colloid Interface Sci. 2020, 560, 519–535. [Google Scholar] [CrossRef]
- Zhen, Y.; Yang, C.; Shen, H.; Xue, W.; Gu, C.; Feng, J.; Zhang, Y.; Fu, F.; Liang, Y. Photocatalytic Performance and Mechanism Insights of a S-Scheme g-C3N4/Bi2MoO6 Heterostructure in Phenol Degradation and Hydrogen Evolution Reactions under Visible Light. Phys. Chem. Chem. Phys. 2020, 22, 26278–26288. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Alsohaimi, I.H.; Betiha, M.A.; Essawy, A.A.; Mousa, A.A.; Alruwaili, S.F.; Hassan, H.M.A. Green Fabrication of Silver Imprinted Titania / Silica Nanospheres as Robust Visible Light-Induced Photocatalytic Wastewater Purification. Mater. Chem. Phys. 2020, 241, 122403. [Google Scholar] [CrossRef]
- Cheng, P.; Zhao, X.; El-Ramady, H.; Elsakhawy, T.; Waigi, M.G.; Ling, W. Formation of Environmentally Persistent Free Radicals from Photodegradation of Triclosan by Metal Oxides/Silica Suspensions and Particles. Chemosphere 2022, 290, 133322. [Google Scholar] [CrossRef]
- Ferreira, O.; Monteiro, O.C.; do Rego, A.M.B.; Ferraria, A.M.; Batista, M.; Santos, R.; Monteiro, S.; Freire, M.; Silva, E.R. Visible Light-Driven Photodegradation of Triclosan and Antimicrobial Activity against Legionella Pneumophila with Cobalt and Nitrogen Co-Doped TiO2 Anatase Nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106735. [Google Scholar] [CrossRef]
- Coelho, F.E.B.; Deemter, D.; Candelario, V.M.; Boffa, V.; Malato, S.; Magnacca, G. Development of a Photocatalytic Zirconia-Titania Ultrafiltration Membrane with Anti-Fouling and Self-Cleaning Properties. J. Environ. Chem. Eng. 2021, 9, 106671. [Google Scholar] [CrossRef]
- Huang, H.; Shi, R.; Zhang, X.; Zhao, J.; Su, C.; Zhang, T. Photothermal-Assisted Triphase Photocatalysis Over a Multifunctional Bilayer Paper. Angew. Chem. Int. Ed. 2021, 60, 22963–22969. [Google Scholar] [CrossRef]
- Mohanty, S.; Babu, P.; Parida, K.; Naik, B. Surface-Plasmon-Resonance-Induced Photocatalysis by Core-Shell SiO2@Ag NCs@Ag3PO4 toward Water-Splitting and Phenol Oxidation Reactions. Inorg. Chem. 2019, 58, 9643–9654. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.A.F.M.; Razali, M.H.; Osman, M.U.; Azam, B.M. Synthesis and Characterisation of TiO2/g-C3N4 as Photocatalyst for Photodegradation of Dyes, Phenol and Caffeine. Adv. Mater. Process. Technol. 2022, 8, 4396–4415. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, H.; Bi, H.; Zhong, G.; Chen, J.; Wu, Y.; Wei, W. Multifunctional Bismuth Oxychloride/Mesoporous Silica Composites for Photocatalysis, Antibacterial Test, and Simultaneous Stripping Analysis of Heavy Metals. ACS Omega 2018, 3, 973–981. [Google Scholar] [CrossRef] [PubMed]









| Geographic Region a | Regulatory Agency | Use and Limitations of TCS | Ref. |
|---|---|---|---|
| European Union | Scientific Committee on Consumer Safety (SCCS) |
| [12] |
| USA | Food and Drug Administration (FDA) |
| [6,13,14] |
| Latin America | Mexican Secretariat of Health |
| [15] |
| Japan | Pharmaceuticals and Medical Devices Agency (PMDA) |
| [13] |
| South Africa | South African Health Products Regulatory Authority (SAHPRA) |
| [16] |
| South Korea | Korean Food and Drug Administration (KFDA) |
| [13] |
| Platform (and Nanomaterial) | Target Pollutant | Additional Functionality | Highlights | Ref. |
|---|---|---|---|---|
| TiO2 (Cobalt and nitrogen co-doped TiO2 anatase nanoparticles) | TCS | Antibacterial against Legionella pneumophila | The material degrades > 99% TCS in 20 min from 10 ppm solution under UV and light emitting diode (LED) light. It can also serve as an antibacterial agent against Legionella pneumophila, Staphylococcus aureus (https://www.sciencedirect.com/topics/medicine-and-dentistry/staphylococcus accessed on 4 September 2023), and Escherichia coli (https://www.sciencedirect.com/topics/medicine-and-dentistry/escherichia accessed on 4 September 2023) | [95] |
| SiO2@TiO2 (SiO2@TiO2 core-shell nanomaterials) | Phenol | Water-splitting | AgCu (in 1:3 mol ratio) deposited on core-shell SiO2@TiO2 hybrid nanomaterials for phenol oxidation and photocatalytic hydrogen generation under visible light. Compared with the monometallic materials, the hybrid material shows two-times stronger catalytic activity toward phenol oxidation and three-times higher photocatalytic activity toward hydrogen generation while producing eight-times greater photocurrent. | [73] |
| SiO2 (Core-Shell SiO2@Ag NCs@Ag3PO4) | Phenol | Water-splitting and phenol oxidation | The material catalyzes water splitting and phenol oxidation up to 91% in 120 min. During water splitting, it catalyzes the hydrogen evolution reaction and the oxygen evolution reaction with rates of 2460 mol h−1 g−1 of H2 and 1236 mol h−1 g−1 of O2, respectively. | [98] |
| TiO2 (TiO2/g-C3N4 as photocatalyst) | Phenol | Photocatalyst for degradation of dyes, phenol, and caffeine. | Under UV light irradiation, the material effectively photocatalyzes the degradation of methylene blue and caffeine, with methylene blue degradation reaching nearly 100% after 240 min and phenol degradation reaching 75% after 300 min. | [99] |
| SiO2 (Bismuth oxychloride/mesoporous silica) | Phenol | Antibacterial, heavy metal stripping analysis. | The composite material shows photocatalytic activity toward the degradation of rhodamine B as well as strong antibacterial activity against Staphylococcus aureus and Enterococcus faecalis. However, the material’s activity for phenol degradation is not as effective as other materials reported in the literature. | [100] |
| TiO2 (Ce-Y-ZrO2/TiO2 on ZrO2/SiC support material fabricated as a membrane) | Phenol, humic acid | Antifouling or self-cleaning | The membrane is effective for photodegrading phenol and humic acid under simulated sunlight irradiation. The membrane also exhibits better anti-fouling (smaller flux decline) and higher permeation flux properties under irradiation compared to filtration in the dark. Moreover, the membrane shows self-cleaning properties upon irradiation, which enables recovery of up to 97% of the original flux. The experiment using commercial TiO2 (P25) results in 100% phenol degradation in 150 min. Although the unsupported membrane (Ce-Y-ZrO2/TiO2) shows a lower activity, resulting in 70% degradation, it is easier to recover and reuse. The fact that the membrane can easily be separated from water systems after being used makes it also more advantageous. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Hernández, M.; Cox, J.; Thomas, B.; Asefa, T. Nanomaterials for Removal of Phenolic Derivatives from Water Systems: Progress and Future Outlooks. Molecules 2023, 28, 6568. https://doi.org/10.3390/molecules28186568
Ramírez-Hernández M, Cox J, Thomas B, Asefa T. Nanomaterials for Removal of Phenolic Derivatives from Water Systems: Progress and Future Outlooks. Molecules. 2023; 28(18):6568. https://doi.org/10.3390/molecules28186568
Chicago/Turabian StyleRamírez-Hernández, Maricely, Jordan Cox, Belvin Thomas, and Tewodros Asefa. 2023. "Nanomaterials for Removal of Phenolic Derivatives from Water Systems: Progress and Future Outlooks" Molecules 28, no. 18: 6568. https://doi.org/10.3390/molecules28186568
APA StyleRamírez-Hernández, M., Cox, J., Thomas, B., & Asefa, T. (2023). Nanomaterials for Removal of Phenolic Derivatives from Water Systems: Progress and Future Outlooks. Molecules, 28(18), 6568. https://doi.org/10.3390/molecules28186568