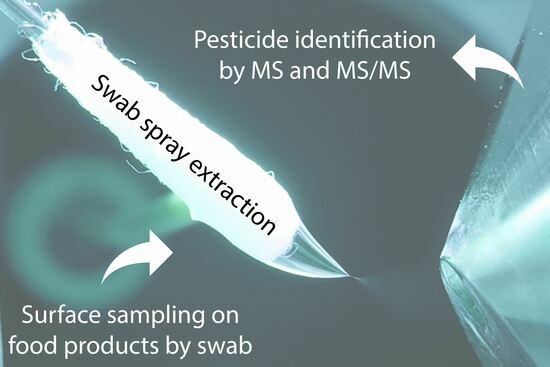
Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry
Abstract
:1. Introduction
1.1. Ambient Ionization
1.2. Swab Spray Ionization
1.3. Pesticide Analysis on Food
2. Results and Discussion
2.1. Swab Background Evaluation
2.2. Qualitative Workflow
2.3. Structure and Solvent Effects on the Formation of Sodium Adducts
2.4. Quantitative Analysis
3. Material and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Swab Spray Handling Procedure
3.4. Evaluation of Quantitation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Huang, M.-Z.; Cheng, S.-C.; Cho, Y.-T.; Shiea, J. Ambient Ionization Mass Spectrometry: A Tutorial. Anal. Chim. Acta 2011, 702, 1–15. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- Rankin-Turner, S.; Reynolds, J.C.; Turner, M.A.; Heaney, L.M. Applications of Ambient Ionization Mass Spectrometry in 2021: An Annual Review. Anal. Sci. Adv. 2022, 3, 67–89. [Google Scholar] [CrossRef]
- Beneito-Cambra, M.; Gilbert-López, B.; Moreno-González, D.; Bouza, M.; Franzke, J.; García-Reyes, J.F.; Molina-Díaz, A. Ambient (Desorption/Ionization) Mass Spectrometry Methods for Pesticide Testing in Food: A Review. Anal. Methods 2020, 12, 4831–4852. [Google Scholar] [CrossRef]
- Chen, R.; Deng, J.; Fang, L.; Yao, Y.; Chen, B.; Wang, X.; Luan, T. Recent Applications of Ambient Ionization Mass Spectrometry in Environmental Analysis. Trends Environ. Anal. Chem. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Forbes, T.P.; Sisco, E. Recent Advances in Ambient Mass Spectrometry of Trace Explosives. Analyst 2018, 143, 1948–1969. [Google Scholar] [CrossRef]
- Boronat Ena, M.D.M.; Cowan, D.A.; Abbate, V. Ambient Ionization Mass Spectrometry Applied to New Psychoactive Substance Analysis. Mass. Spectrom. Rev. 2023, 42, 3–34. [Google Scholar] [CrossRef]
- Li, L.-H.; Hsieh, H.-Y.; Hsu, C.-C. Clinical Application of Ambient Ionization Mass Spectrometry. Mass Spectrom. 2017, 6, S0060. [Google Scholar] [CrossRef]
- Burns, D.; Mathias, S.; McCullough, B.J.; Hopley, C.J.; Douce, D.; Lumley, N.; Bajic, S.; Sears, P. Ambient Ionisation Mass Spectrometry for the Trace Detection of Explosives Using a Portable Mass Spectrometer. Int. J. Mass Spectrom. 2022, 471, 116735. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Yannell, K.E.; Jarmusch, A.K.; Pirro, V.; Ouyang, Z.; Cooks, R.G. Ambient Ionization Mass Spectrometry for Point-of-Care Diagnostics and Other Clinical Measurements. Clin. Chem. 2016, 62, 99–110. [Google Scholar] [CrossRef]
- Pirro, V.; Jarmusch, A.K.; Vincenti, M.; Cooks, R.G. Direct Drug Analysis from Oral Fluid Using Medical Swab Touch Spray Mass Spectrometry. Anal. Chim. Acta 2015, 861, 47–54. [Google Scholar] [CrossRef]
- Pirro, V.; Llor, R.S.; Jarmusch, A.K.; Alfaro, C.M.; Cohen-Gadol, A.A.; Hattab, E.M.; Cooks, R.G. Analysis of Human Gliomas by Swab Touch Spray-Mass Spectrometry: Applications to Intraoperative Assessment of Surgical Margins and Presence of Oncometabolites. Analyst 2017, 142, 4058–4066. [Google Scholar] [CrossRef] [PubMed]
- Jarmusch, A.K.; Pirro, V.; Logsdon, D.L.; Cooks, R.G. Direct Ion Generation from Swabs. Talanta 2018, 184, 356–363. [Google Scholar] [CrossRef]
- Morato, N.M.; Pirro, V.; Fedick, P.W.; Cooks, R.G. Quantitative Swab Touch Spray Mass Spectrometry for Oral Fluid Drug Testing. Anal. Chem. 2019, 91, 7450–7457. [Google Scholar] [CrossRef]
- Fedick, P.W.; Bain, R.M. Swab Touch Spray Mass Spectrometry for Rapid Analysis of Organic Gunshot Residue from Human Hand and Various Surfaces Using Commercial and Fieldable Mass Spectrometry Systems. Forensic Chem. 2017, 5, 53–57. [Google Scholar] [CrossRef]
- Bain, R.M.; Fedick, P.W.; Dilger, J.M.; Cooks, R.G. Analysis of Residual Explosives by Swab Touch Spray Ionization Mass Spectrometry. Propellants Explos. Pyrotech. 2018, 43, 1139–1144. [Google Scholar] [CrossRef]
- Costa, C.; van Es, E.M.; Sears, P.; Bunch, J.; Palitsin, V.; Cooper, H.; Bailey, M.J. Exploring a Route to a Selective and Sensitive Portable System for Explosive Detection- Swab Spray Ionisation Coupled to of High-Field Assisted Waveform Ion Mobility Spectrometry (FAIMS). Forensic Sci. Int. 2019, 1, 214–220. [Google Scholar] [CrossRef]
- Jarmusch, A.K.; Pirro, V.; Kerian, K.S.; Cooks, R.G. Detection of Strep Throat Causing Bacterium Directly from Medical Swabs by Touch Spray-Mass Spectrometry. Analyst 2014, 139, 4785–4789. [Google Scholar] [CrossRef]
- Yang, B.; Wang, F.; Yang, X.; Zou, W.; Wang, J.; Zou, Y.; Liu, F.; Liu, H.; Huang, O. Medical Swab Touch Spray-Mass Spectrometry for Newborn Screening of Nicotine and Cotinine in Meconium. J. Mass Spectrom. 2016, 51, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.T.; Szalwinski, L.J.; Schrader, R.L.; Pirro, V.; Hilger, R.; Cooks, R.G. Precursor and Neutral Loss Scans in an RF Scanning Linear Quadrupole Ion Trap. J. Am. Soc. Mass Spectrom. 2018, 29, 1345–1354. [Google Scholar] [CrossRef]
- Statistical Yearbook 2021 World Food and Agriculture. Available online: www.Fao.Org/3/Cb4477en/Cb4477en.Pdf (accessed on 13 March 2023).
- d’Hose, D.; Isenborghs, P.; Brusa, D.; Jordan, B.F.; Gallez, B. The Short-Term Exposure to SDHI Fungicides Boscalid and Bixafen Induces a Mitochondrial Dysfunction in Selective Human Cell Lines. Molecules 2021, 26, 5842. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of Pesticides: A Brief Review. Front. Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y.; El-Nahhal, I. Cardiotoxicity of Some Pesticides and Their Amelioration. Environ. Sci. Pollut. Res. 2021, 28, 44726–44754. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, J.; Xu, Y.; Liu, H.; Yang, G.; He, Y.; Xu, J.; Lu, Z. Suspecting Screening “Known Unknown” Pesticides and Transformation Products in Soil at Pesticide Manufacturing Sites. Sci. Total Environ. 2022, 808, 152074. [Google Scholar] [CrossRef]
- Khetagoudar, M.C.; Chetti, M.B.; Raghu, A.V.; Bilehal, D.C. Multiresidue Pesticide Analysis in Cabbage and Cauliflower Using Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS). In Pesticides in Crop Production; Wiley: Hoboken, NJ, USA, 2020; pp. 221–231. [Google Scholar]
- Wong, J.W.; Wang, J.; Chow, W.; Carlson, R.; Jia, Z.; Zhang, K.; Hayward, D.G.; Chang, J.S. Perspectives on Liquid Chromatography—High-Resolution Mass Spectrometry for Pesticide Screening in Foods. J. Agric. Food Chem. 2018, 66, 9573–9581. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Moreno-González, D. Current Role of Mass Spectrometry in the Determination of Pesticide Residues in Food. Separations 2022, 9, 148. [Google Scholar] [CrossRef]
- Gormez, E.; Golge, O.; González-Curbelo, M.Á.; Kabak, B. Pesticide Residues in Mandarins: Three-Year Monitoring Results. Molecules 2023, 28, 5611. [Google Scholar] [CrossRef]
- Kang, S.-M.; Won, J.-H.; Han, J.-E.; Kim, J.-H.; Kim, K.-H.; Jeong, H.-I.; Sung, S.-H. Chromatographic Method for Monitoring of Pesticide Residues and Risk Assessment for Herbal Decoctions Used in Traditional Korean Medicine Clinics. Molecules 2023, 28, 3343. [Google Scholar] [CrossRef]
- Fan, X.; Tang, T.; Du, S.; Sang, N.; Huang, H.; Zhang, C.; Zhao, X. Simultaneous Determination of 108 Pesticide Residues in Three Traditional Chinese Medicines Using a Modified QuEChERS Mixed Sample Preparation Method and HPLC-MS/MS. Molecules 2022, 27, 7636. [Google Scholar] [CrossRef]
- Constantinou, M.; Louca-Christodoulou, D.; Agapiou, A. Method Validation for the Determination of 314 Pesticide Residues Using Tandem MS Systems (GC–MS/MS and LC-MS/MS) in Raisins: Focus on Risk Exposure Assessment and Respective Processing Factors in Real Samples (a Pilot Survey). Food Chem. 2021, 360, 129964. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, N.R.; Pallavi, M.S.; Bheemanna, M.; PavanKumar, K.; Chandra Sekhara Reddy, V.; Udaykumar, N.R.; Paramasivam, M.; Yadav, S. Simultaneous Determination of 79 Pesticides in Pigeonpea Grains Using GC–MS/MS and LC–MS/MS. Food Chem. 2021, 347, 128986. [Google Scholar] [CrossRef] [PubMed]
- Stachniuk, A.; Szmagara, A.; Czeczko, R.; Fornal, E. LC-MS/MS Determination of Pesticide Residues in Fruits and Vegetables. J. Environ. Sci. Health Part B 2017, 52, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, Z.; Huang, S.; Wang, J. Non-Targeted Screening of Pesticides for Food Analysis Using Liquid Chromatography High-Resolution Mass Spectrometry—A Review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2020, 37, 1180–1201. [Google Scholar] [CrossRef]
- Yun, H.Y.; Won, E.-J.; Choi, J.; Cho, Y.; Lim, D.-J.; Kim, I.-S.; Shin, K.-H. Stable Isotope Analysis of Residual Pesticides via High Performance Liquid Chromatography and Elemental Analyzer–Isotope Ratio Mass Spectrometry. Molecules 2022, 27, 8587. [Google Scholar] [CrossRef]
- Thurman, E.M.; Ferrer, I.; Zweigenbaum, J.A.; García-Reyes, J.F.; Woodman, M.; Fernández-Alba, A.R. Discovering Metabolites of Post-Harvest Fungicides in Citrus with Liquid Chromatography/Time-of-Flight Mass Spectrometry and Ion Trap Tandem Mass Spectrometry. J. Chromatogr. A 2005, 1082, 71–80. [Google Scholar] [CrossRef]
- Jabot, C.; Daniele, G.; Giroud, B.; Tchamitchian, S.; Belzunces, L.P.; Casabianca, H.; Vulliet, E. Detection and Quantification of Boscalid and Its Metabolites in Honeybees. Chemosphere 2016, 156, 245–251. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium Adduct Formation Efficiency in ESI Source. J. Mass Spectrom. 2013, 48, 695–702. [Google Scholar] [CrossRef]
- Pesticide Residue(s) and Maximum Residue Levels (mg/kg). Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls/details?lg_code=EN&pest_res_id_list=244 (accessed on 13 September 2023).
- CFR—Code of Federal Regulations Title 21. Available online: www.Accessdata.Fda.Gov/Scripts/Cdrh/Cfdocs/Cfcfr/CFRSearch.Cfm?Fr=172.210 (accessed on 9 June 2023).
- Jin, X.; Zhang, S.; Yang, J.; Li, Y. The Potential of Alkyl Polyglucosides and Polyethylene Glycol Glucosides Coatings for Improving the Storage and Shelf-Life of Pineapples. Tenside Surfactants Deterg. 2004, 41, 109–112. [Google Scholar] [CrossRef]







| Compound/Surface | Smear (Relative Signal Intensity) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Azoxystrobin on tomato skin | 72% | 16% | 12% |
| Imazalil on orange peel | 33% | 28% | 38% |
| 5000 ng imazalil on glass | 76% | 12% | 11% |
| 500 ng imazalil on glass | 68% | 20% | 12% |
| 50 ng imazalil on glass | 41% | 38% | 21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muggli, T.M.; Schürch, S. Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry. Molecules 2023, 28, 6611. https://doi.org/10.3390/molecules28186611
Muggli TM, Schürch S. Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry. Molecules. 2023; 28(18):6611. https://doi.org/10.3390/molecules28186611
Chicago/Turabian StyleMuggli, Thomas Michael, and Stefan Schürch. 2023. "Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry" Molecules 28, no. 18: 6611. https://doi.org/10.3390/molecules28186611
APA StyleMuggli, T. M., & Schürch, S. (2023). Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry. Molecules, 28(18), 6611. https://doi.org/10.3390/molecules28186611