Photoprotective Effects of Processed Ginseng Leaf Administration against UVB-Induced Skin Damage in Hairless Mice
Abstract
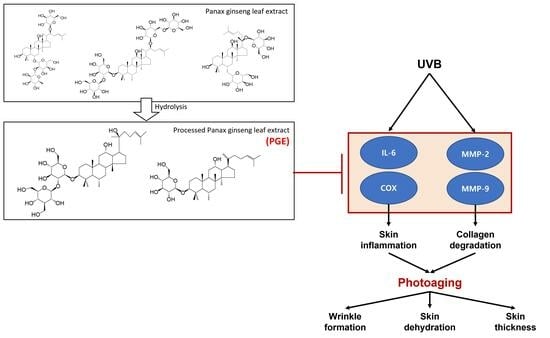
:1. Introduction
2. Results and Discussion
2.1. Examination of PGL and Ginsenoside Content
2.2. Protective Effects of PGL against UVB-Induced Damage in HaCaT Cells
2.3. Body Weight Change
2.4. Effect of PGL on Wrinkle Formation Induced by UV Irradiation
2.5. Effect of PGL on Epidermal Thickness of the Dorsal Skin of Mice Exposed to UV
2.6. Effect of PGL on Transepidermal Water Loss (TEWL) in the Dorsal Skin of Mice Exposed to UV
2.7. Effect of PGL on UVB-Induced Expression of Wrinkle-Related Genes
2.8. Effect of PGL on UVB-Induced Inflammation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Processed Ginseng Leaf Extract (PGL)
3.3. Analytical Conditions
3.4. Cell Culture and UVB Irradiation
3.5. Cytotoxicity Assay
3.6. Evaluation of MMP-2 and MMP-9 Secretion
3.7. Experimental Animals
3.8. PGL Treatment and Experimental Design
3.9. UV Irradiation and Body Weight
3.10. Skin Wrinkles and TEWL
3.11. RT-PCR Analysis
3.12. Histological Observation of Skin
3.13. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.P.; Han, J.X.; Jiang, C.P.; Zhang, Y. Biomarkers oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Csh Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Durr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Ma, W.; Wlaschek, M.; Tantcheva-Poór, L.A.; Schneider, L.; Naderi, Z.; Razi-Wolf, J.; Schuller, K.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connetive tissue. Clin. Exp. Dermatol. 2001, 7, 592–599. [Google Scholar] [CrossRef]
- Steven, D. S Matrix metalloproteinase degradation of extracellular matrix: Biological consequences. Curr. Opin. Cell Biol. 1998, 10, 602–608. [Google Scholar]
- Maipas, S.; Nicolopoulou-Stamati, P. Sun lotion chemicals as endocrine disruptors. Horm-Int. J. Endocrinol. 2015, 14, 32–46. [Google Scholar]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.L.; Ji, L.Y.; Zhang, Y.H.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simoes, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Kim, M.R.; Lee, H.S.; Choi, H.S.; Kim, S.Y.; Park, Y.; Suh, H.J. Protective effects of ginseng leaf extract using enzymatic extraction against oxidative damage of UVA-irradiated human keratinocytes. Appl. Biochem. Biotech. 2014, 173, 933–945. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.S.; Song, M.S.; Seo, H.S.; Moon, C.; Kim, J.C.; Jo, S.K.; Jang, J.S.; Kim, S.H. Photoprotective effect of red ginseng against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Phytother. Res. 2009, 23, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Oral photoprotection: Effective agents and potential candidates. Front. Med. 2018, 5, 188. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Jeon, B.S.; Bock, J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer). Food Chem. 2002, 79, 105–111. [Google Scholar] [CrossRef]
- Bai, Y.P.; Ganzle, M.G. Conversion of ginsenosides by Lactobacillus plantarum studied by liquid chromatography coupled to quadrupole trap mass spectrometry. Food Res. Int. 2015, 76, 709–718. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Yin, G.; Wang, J.; Wang, P.; Chen, Z.Y.; Wang, T.; Ren, G. Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother. Res. 2020, 34, 1226–1236. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, S.; Zhao, L.; Yang, X.; Yao, Y.; Hou, Z.; Xue, P. Stem-leaves of Panax as a rich and sustainable source of less-polar ginsenosides: Comparison of ginsenosides from Panax ginseng, American ginseng and Panax notoginseng prepared by heating and acid treatment. J. Ginseng Res. 2021, 45, 163–175. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Xie, G.; Liu, H.X.; Tan, G.L.; Li, L.; Liu, W.L.; Li, M. Fermented ginseng leaf enriched with rare ginsenosides relieves exercise-induced fatigue via regulating metabolites of muscular interstitial fluid, satellite cells-mediated muscle repair and gut microbiota. J. Funct. Foods 2021, 83, 104509. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, S.Y.; Kim, M.R.; Suh, H.J.; Park, H.J. Changes of ginsenosides in Korean red ginseng (Panax ginseng) fermented by Lactobacillus plantarum M1. Process. Biochem. 2010, 45, 1319–1324. [Google Scholar] [CrossRef]
- Kim, I.W.; Sun, W.S.; Yun, B.S.; Kim, N.R.; Min, D.; Kim, S.K. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J. Ginseng Res. 2013, 37, 124–134. [Google Scholar] [CrossRef]
- Jeong, G.T.; Kim, S.K.; Oh, B.R. Production of fermentable sugars from Chlorella sp. by solid-acid catalyst. Algal. Res. 2020, 51, 102044. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, J.; Liang, W.X.; Yao, L.; Gao, W.Y. Promotion of ginsenosides production in a co-cultivation system of Panax ginseng adventitious roots and immobilized Aspergillus niger. Ind. Crop. Prod. 2019, 140, 111564. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lee, H.S.; Jung, E.Y.; Han, S.H.; Park, Y.; Suh, H.J. Photoprotective effects of topical ginseng leaf extract using Ultraflo L against UVB-induced skin damage in hairless mice. J. Ginseng Res. 2017, 41, 456–462. [Google Scholar] [CrossRef]
- Jo, S.K.; Kim, I.S.; Yoon, K.S.; Yoon, H.H.; Yoo, H.H. Preparation of ginsenosides Rg3, Rk1, and Rg5-selectively enriched ginsengs by a simple steaming process. Eur. Food Res. Technol. 2015, 240, 251–256. [Google Scholar] [CrossRef]
- Kang, O.J.; Kim, J.-S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng C.A. Mayer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef]
- Yoon, S.J.; Park, J.Y.; Choi, S.; Lee, J.B.; Jung, H.; Kim, T.D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef]
- Matsumura, Y.; Anathaswamy, H. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Ritte, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 25, 335–339. [Google Scholar] [CrossRef]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Derm. Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Moriwaki, S.; Fujimura, T.; Takema, Y. Inhibitory effect of an extract of Sanguisorba officinalis L. on ultraviolet-B-induced photodamage of rat skin. Biol. Pharm. Bull. 2001, 24, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Grundmann-Kollmann, M. Low molecular weight antioxidants and their role in skin ageing. Clin. Exp. Dermatol. 2001, 26, 578–582. [Google Scholar] [CrossRef]







| Content (mg/g) | ||
|---|---|---|
| Processed Ginseng Leaf Extract | Ginseng Leaf Extract | |
| Rg1 | 10.5 ± 3.30 | 74.2 ± 2.79 |
| Re | 30.00 ± 0.85 | 167.7 ± 1.24 |
| Rg2 | 39.3 ± 1.77 | 32.4 ± 0.98 |
| Rb1 | 4.9 ± 0.06 | 15.6 ± 0.17 |
| Rc | 11.30 ± 0.01 | 37.2 ± 0.21 |
| Rb2 | 23.5 ± 1.98 | 54.0 ± 2.07 |
| Rb3 | 5.0 ± 0.11 | 12.6 ± 0.16 |
| Rd | 83.7 ± 2.31 | 125.1 ± 4.19 |
| Rg3 | 29.4 ± 1.50 | 2.1 ± 0.00 |
| Rk1 | 35.2 ± 1.76 | - |
| Gene | Primer Sequence | |
|---|---|---|
| MMP-2 | Forward | 5′-CAG GGA ATG AGT ACT GGG TCT ATT-3′ |
| Reverse | 5′-ACT CCA GTT AAA GGC AGC ATC TAC-3′ | |
| MMP-9 | Forward | 5′-AAT CTC TTC TAG AGA CTG GGA AGG AG-3′ |
| Reverse | 5′-AGC TGA TTG ACT AAA GTA GCT GGA-3′ | |
| IL-6 | FAM | 5′-CTGTGTAATGAAAGACGGCACACCCACC-3′ |
| COX-2 | Forward | 5′-ATG GAT CGA AGA CTA CGT GCA A-3′ |
| Reverse | 5′-GGG ATT TCC CAT AAG TCC TTT C-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, E.; Lee, Y.M.; Kim, S.-H.; Kim, D.-S. Photoprotective Effects of Processed Ginseng Leaf Administration against UVB-Induced Skin Damage in Hairless Mice. Molecules 2023, 28, 6734. https://doi.org/10.3390/molecules28186734
Son E, Lee YM, Kim S-H, Kim D-S. Photoprotective Effects of Processed Ginseng Leaf Administration against UVB-Induced Skin Damage in Hairless Mice. Molecules. 2023; 28(18):6734. https://doi.org/10.3390/molecules28186734
Chicago/Turabian StyleSon, Eunjung, Yun Mi Lee, Seung-Hyung Kim, and Dong-Seon Kim. 2023. "Photoprotective Effects of Processed Ginseng Leaf Administration against UVB-Induced Skin Damage in Hairless Mice" Molecules 28, no. 18: 6734. https://doi.org/10.3390/molecules28186734
APA StyleSon, E., Lee, Y. M., Kim, S. -H., & Kim, D. -S. (2023). Photoprotective Effects of Processed Ginseng Leaf Administration against UVB-Induced Skin Damage in Hairless Mice. Molecules, 28(18), 6734. https://doi.org/10.3390/molecules28186734