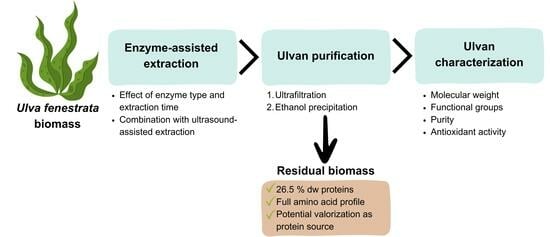
Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata
Abstract
:1. Introduction
2. Results
2.1. Ulva fenestrata Biochemical Composition
2.2. Ulvan Extraction
2.2.1. Enzyme-Assisted Extraction: Effect of Extraction Time
2.2.2. Combined Enzymatic and Ultrasound Extraction
2.3. Characterization of Extracted Ulvan
2.3.1. Molecular Weight Distribution
2.3.2. Purity and Total Antioxidant Capacity
2.3.3. ATR-FTIR Analysis
2.4. Potential Valorization of the Residual Biomass
3. Materials and Methods
3.1. Macroalgae Biomass
3.2. Biomass Biochemical Characterization
3.2.1. Proteins and Amino Acids Distribution
3.2.2. Lipids
3.2.3. Ash
3.3. Ulvan Extraction and Purification
3.3.1. Enzyme Activity Assays
3.3.2. Enzyme-Assisted Extraction (EAE)
3.3.3. Combined Enzymatic–Ultrasound Extraction (U-EAE) and Ultrasound-Assisted Extraction (UAE)
3.4. Ulvan Characterization
3.4.1. Gel Permeation Chromatography
3.4.2. Total Phenolic Compounds
3.4.3. Total Antioxidant Capacity
3.4.4. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Commission. CORDIS Results Pack on Algae. Discovering Algae’s Power as a Renewable Resource; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; EUR-Lex: Brussels, Belgium, 2021; p. 240. [Google Scholar]
- Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.G.; Abdullah, C.K.; Ikramullah; Yahya, E.B.; Chong, E.W.N.; Abdul Khalil, H.P.S. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rosas, J.; Cruz-Galvez, A.M.; Gomez-Aldapa, C.A.; Falfan-Cortes, R.N.; Guzman-Ortiz, F.A.; Rodríguez-Marín, M.L. Biopolymer films and the effects of added lipids, nanoparticles and antimicrobials on their mechanical and barrier properties: A review. Int. J. Food Sci. Technol. 2016, 51, 1967–1978. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Z.; Yao, L.; Feng, T.; Song, S.; Sun, M. Insights into the Edible and Biodegradable Ulvan-Based Films and Coatings for Food Packaging. Foods 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Richel, A.; Blecker, C.; Boufi, S.; Attia, H.; Garna, H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019, 135, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Guidara, M.; Yaich, H.; Benelhadj, S.; Adjouman, Y.D.; Richel, A.; Blecker, C.; Sindic, M.; Boufi, S.; Attia, H.; Garna, H. Smart ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties. Int. J. Biol. Macromol. 2020, 150, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Ramu Ganesan, A.; Shanmugam, M.; Bhat, R. Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef]
- Davoodi, M.N.; Milani, J.M.; Farahmandfar, R. Preparation and characterization of a novel biodegradable film based on sulfated polysaccharide extracted from seaweed Ulva intestinalis. Food Sci. Nutr. 2021, 9, 4108–4116. [Google Scholar] [CrossRef]
- Don, T.-M.; Liu, L.-M.; Chen, M.; Huang, Y.-C. Crosslinked complex films based on chitosan and ulvan with antioxidant and whitening activities. Algal Res. 2021, 58, 102423. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Lang, M.; Terme, N.; Bourgougnon, N.; Bedoux, G. Production of Active Poly- and Oligosaccharidic Fractions from Ulva sp. by Combining Enzyme-Assisted Extraction (EAE) and Depolymerization. Metabolites 2019, 9, 182. [Google Scholar] [CrossRef]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Da Borges Silva, E.A.; Reis, R.L.; Rodrigues, A.E. Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Herrera Barragán, J.A.; Olivieri, G.; Boboescu, I.; Eppink, M.; Wijffels, R.; Kazbar, A. Enzyme assisted extraction for seaweed multiproduct biorefinery: A techno-economic analysis. Front. Mar. Sci. 2022, 9, 948086. [Google Scholar] [CrossRef]
- Huck, W. Resolution adopted by the General Assembly on 25 September 2015. In Sustainable Development Goals; Huck, W., Ed.; Nomos Verlagsgesellschaft mbH & Co. KG: Baden-Baden, Germany, 2022; pp. 653–684. ISBN 9783748902065. [Google Scholar]
- Stedt, K.; Steinhagen, S.; Trigo, J.P.; Kollander, B.; Undeland, I.; Toth, G.B.; Wendin, K.; Pavia, H. Post-harvest cultivation with seafood process waters improves protein levels of Ulva fenestrata while retaining important food sensory attributes. Front. Mar. Sci. 2022, 9, 991359. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Oerbekke, A.; Cvijetinovic, S.; Wahlström, N.; Harrysson, H.; Steinhagen, S.; Kinnby, A.; White, J.; Edlund, U.; et al. Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3255–3263. [Google Scholar] [CrossRef]
- Steinhagen, S.; Enge, S.; Larsson, K.; Olsson, J.; Nylund, G.M.; Albers, E.; Pavia, H.; Undeland, I.; Toth, G.B. Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm. JMSE 2021, 9, 615. [Google Scholar] [CrossRef]
- Steinhagen, S.; Larsson, K.; Olsson, J.; Albers, E.; Undeland, I.; Pavia, H.; Toth, G.B. Closed life-cycle aquaculture of sea lettuce (Ulva fenestrata): Performance and biochemical profile differ in early developmental stages. Front. Mar. Sci. 2022, 9, 942679. [Google Scholar] [CrossRef]
- Magnusson, M.; Glasson, C.R.; Vucko, M.J.; Angell, A.; Neoh, T.L.; Nys, R.d. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Res. 2019, 41, 101555. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Kumar Suryawanshi, R.; Agrawal, S.; Ghosh, M.; Kango, N. Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresour. Technol. 2018, 250, 733–740. [Google Scholar] [CrossRef]
- Fernandes, V.O.; Costa, M.; Ribeiro, T.; Serrano, L.; Cardoso, V.; Santos, H.; Lordelo, M.; Ferreira, L.; Fontes, C. 1,3-1,4-β-Glucanases and not 1,4-β-glucanases improve the nutritive value of barley-based diets for broilers. Anim. Feed. Sci. Technol. 2016, 211, 153–163. [Google Scholar] [CrossRef]
- Eberhardt, A.; López, E.C.; Marino, F.; Mammarella, E.J.; Manzo, R.M.; Sihufe, G.A. Whey protein hydrolysis with microbial proteases: Determination of kinetic parameters and bioactive properties for different reaction conditions. Int. J. Dairy Technol. 2021, 74, 489–504. [Google Scholar] [CrossRef]
- Da Rosa, L.O.L.; Santana, M.C.; Avezedo, T.L.; Brígida, A.I.S.; Godoy, R.; Pacheco, S.; Mellinger-Silva, C.; Cabral, L.M.C. A comparison of dual-functional whey hydrolysates by the use of commercial proteases. Food Sci. Technol. 2018, 38, 31–36. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, W.; Gan, J.; Li, Y.; Pan, Y.; Li, J.; Chen, H. Physicochemical properties and anti-oxidation activities of ulvan from Ulva pertusa Kjellm. Algal Res. 2021, 55, 102269. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Anisha, G.S.; Augustianath, T.; Padmakumari, S.; Singhania, R.R.; Pandey, A.; Patel, A.K. Ulvan from green macroalgae: Bioactive properties advancing tissue engineering, drug delivery systems, food industry, agriculture and water treatment. Bioresour. Technol. Rep. 2023, 22, 101457. [Google Scholar] [CrossRef]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef]
- Ben Amor, C.; Jmel, M.A.; Chevallier, P.; Mantovani, D.; Smaali, I. Efficient extraction of a high molecular weight ulvan from stranded Ulva sp. biomass: Application on the active biomembrane synthesis. Biomass Conv. Bioref. 2023, 13, 3975–3985. [Google Scholar] [CrossRef]
- Kazemi, M.; Fathi, M.; Jahanbin, K.; Taghdir, M.; Abbaszadeh, S. Optimization of ultrasonic-assisted hot acidic solvent extraction of ulvan from Ulva intestinalis of the Persian Gulf: Evaluation of structural, techno-functional, and bioactivity properties. Food Hydrocoll. 2023, 142, 108837. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-fouling Properties. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Sassi, J.-F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal Variability of Physicochemical and Rheological Properties of Ulvan in two Ulva Species (Chlorophyta) from the Brittany Coast. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.; Sims, I.M.; Carnachan, S.M.; Nys, R.d.; Magnusson, M. A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Gomaa, M.; Al-Badaani, A.A.; Hifney, A.F.; Adam, M.S. Utilization of cellulose and ulvan from the green seaweed Ulva lactuca in the development of composite edible films with natural antioxidant properties. J. Appl. Phycol. 2022, 34, 2615–2626. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Maehre, H.K.; Malde, M.K.; Eilertsen, K.-E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Mandalka, A.; Cavalcanti, M.I.L.G.; Harb, T.B.; Toyota Fujii, M.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods 2022, 11, 1201. [Google Scholar] [CrossRef]
- Lamp, A.; Kaltschmitt, M.; Lüdtke, O. Improved HPLC-method for estimation and correction of amino acid losses during hydrolysis of unknown samples. Anal. Biochem. 2018, 543, 140–145. [Google Scholar] [CrossRef]
- Lamp, A. Proteingewinnung aus Bioethanolschlempe; Hamburg University of Technology: Hamburg, Germany, 2020. [Google Scholar]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Americ. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- DIN EN ISO 18122; Biogene Festbrennstoffe – Bestimmung des Aschegehaltes. Beuth: Berlin, Germany, 2015.
- O’ Brien, R.; Hayes, M.; Sheldrake, G.; Tiwari, B.; Walsh, P. Macroalgal Proteins: A Review. Foods 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Pio, L.B.; Bule, P.; Cardoso, V.A.; Duarte, M.; Alfaia, C.M.; Coelho, D.F.; Brás, J.A.; Fontes, C.M.G.A.; Prates, J.A.M. Recalcitrant cell wall of Ulva lactuca seaweed is degraded by a single ulvan lyase from family 25 of polysaccharide lyases. Anim. Nutr. 2022, 9, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Islam, F.; Roy, N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res. Notes 2018, 11, 445. [Google Scholar] [CrossRef]
- Secades, P.; Guijarro, J.A. Purification and Characterization of an Extracellular Protease from the Fish Pathogen Yersinia ruckeri and Effect of Culture Conditions on Production. Appl. Environ. Microbiol. 1999, 65, 3969–3975. [Google Scholar] [CrossRef]
- Aguilar, J.G.d.S.; Granato Cason, V.; de Castro, R.J.S. Improving antioxidant activity of black bean protein by hydrolysis with protease combinations. Int. J. Food Sci. Technol. 2019, 54, 34–41. [Google Scholar] [CrossRef]
- Hardouin, K.; Burlot, A.-S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Malvis Romero, A.; Brozio, F.; Kammler, S.; Burkhardt, C.; Baruth, L.; Kaltschmitt, M.; Antranikian, G.; Liese, A. Enzyme-Assisted Extraction of Alginate from Beach Wrack Fucus vesiculosus. Chem. Ing. Tech. 2023, 95, 549–556. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Massironi, A.; Puppi, D.; Creti, D.; Domingo Martinez, E.; Bonistalli, C.; Fabroni, C.; Morgenni, F.; Chiellini, F. Development of ulvan-based emulsions containing flavour and fragrances for food and cosmetic applications. Flavour. Fragr. J. 2019, 34, 411–425. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT—Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]







| Amino Acid | Amino Acid Distribution (% of TAA) | |
|---|---|---|
| Ulva fenestrata | WHO Requirement (% EAA in Total Protein) | |
| Aspartic acid | 15.1 ± 0.06 | |
| Glutamic acid | 12.1 ± 0.09 | |
| Serine | 5.86 ± 0.08 | |
| Histidine * | 2.74 ± 0.03 | 1.5 |
| Glycine | 6.21 ± 0.04 | |
| Threonine * | 5.42 ± 0.05 | 2.3 |
| Arginine | 5.52 ± 0.06 | |
| Alanine | 9.59 ± 0.03 | |
| Tyrosine | 3.02 ± 0.06 | |
| Valine * | 6.29 ± 0.07 | 3.9 |
| Methionine * | 1.72 ± 0.01 | 1.6 |
| Phenylalanine * | 6.38 ± 0.004 | 3.8 |
| Isoleucine * | 4.39 ± 0.07 | 3 |
| Leucine * | 7.39 ± 0.02 | 5.9 |
| Lysine * | 4.57 ± 0.01 | 4.5 |
| Proline | 3.75 ± 0.03 | |
| Total protein content (% dw) | 19.8 ± 1.31 | |
| Total essential amino acids (% dw) | 38.9 ± 0.04 | |
| Enzyme | TPCs, g kg−1 | Proteins, g kg−1 | TAC, g kg−1 |
|---|---|---|---|
| Viscozyme L | 0.25 ± 0.024 | 24.5 ± 4.50 | 131 ± 16.1 |
| Cellulysin | 0.16 ± 0.026 | 62.5 ± 7.05 | 197 ± 94.5 |
| Neutrase 0.8L | 0.13 ± 0.034 | 130 ± 15 | 106 ± 21.9 |
| Flavourzyme | 0.16 ± 0.023 | 81.5 ± 29.4 | 115 ± 40.5 |
| Band | Wavenumber (cm−1) | Functional Group |
|---|---|---|
| a | 3500–3000 | –OH; N–H |
| b | 2970 | C–H |
| c | 2930 | C–H |
| d | 1720 | C=O |
| e | 1600 | C=O; C=C (arom.); –N–H |
| f | 1420 | COO− |
| g | 1380 | –CH3 |
| h | 1260 | S=O |
| i | 1215 | C–O; C–N |
| j | 1045 | C–O |
| k | 980 | C–O–C |
| l | 840 | C–O–S |
| Residual Biomass | Protein Content,% dw | EAA Content,% dw |
|---|---|---|
| Cellulysin | 26.5 | 40.6 |
| Neutrase 0.8L | 18.3 | 40.9 |
| U-EAE | 23.7 | 40.4 |
| Raw biomass | 19.8 ± 1.31 | 38.9 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvis Romero, A.; Picado Morales, J.J.; Klose, L.; Liese, A. Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata. Molecules 2023, 28, 6781. https://doi.org/10.3390/molecules28196781
Malvis Romero A, Picado Morales JJ, Klose L, Liese A. Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata. Molecules. 2023; 28(19):6781. https://doi.org/10.3390/molecules28196781
Chicago/Turabian StyleMalvis Romero, Ana, José Julián Picado Morales, Leon Klose, and Andreas Liese. 2023. "Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata" Molecules 28, no. 19: 6781. https://doi.org/10.3390/molecules28196781
APA StyleMalvis Romero, A., Picado Morales, J. J., Klose, L., & Liese, A. (2023). Enzyme-Assisted Extraction of Ulvan from the Green Macroalgae Ulva fenestrata. Molecules, 28(19), 6781. https://doi.org/10.3390/molecules28196781