Wound Healing Properties of Natural Products: Mechanisms of Action
Abstract
:1. Introduction
2. Wound Healing Process
2.1. Hemostasis
2.2. Inflammatory Phase
2.3. Proliferative Phase
2.4. Remodeling Phase
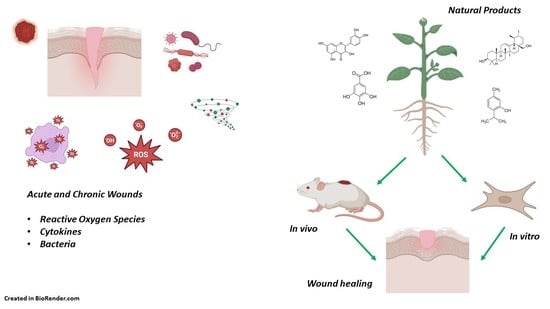
3. In Vitro Studies on the Effect of Natural Products on Wound Healing
4. In Vivo Studies on the Effect of Natural Products on the Wound Healing Process
| Source | Main Components | Model | Treatment | Key Results | Type of Lesion | Reference |
|---|---|---|---|---|---|---|
| Fraxinus angustifolia | Rutin, quercetin, tannic acid, catechin | Male CD-1 mice (5–6 weeks old, 25–35 g) | Cutaneous inflammation was induced by phorbol ester. Polyphenol extract was incorporated into liposome-like vesicles and applied | Leaf-loaded EG-PEVs reduced edema size and myeloperoxidase activity | Chronic | [79] |
| Bletilla striata | Protocatechuic acid, p-hydroxybenzoic acid, caffeic acid, p-hydroxy- benzaldehyde, 3-hydroxycinnamic acid, and ferulic acid | Outbred male mice (3–8 weeks old, 18–22 g) | One mL of Bletilla ointment was applied to burn mice 2 times per day for 15 days | The Bletilla ointment-treated mice improved the wound healing rapidly by day 5 and almost fully healed by day 13 | Acute | [80] |
| Artemisia judaica | Piperitone, terpinene-4-ol, α-thujone, β-thujone, 1,8-cineole, camphor, linalool | A skin burn induction model was used on Sprague Dawley female rats (150 g, 3 months old) | Ointment with 5% A. judaica essential oils applied twice per day to rats with second degree burns for 21 days | The A. judaica ointment improved the wound healing process by increasing SOD and CAT levels, reduced the pro-inflammatory marker TNF-α and increased the anti-inflammatory TGF-b1 and IL-10 levels | Acute | [81] |
| Mentha longifolia subs. Typhoides and schimperi | Subsp. typhoides: piperitenone oxide, piperitone oxide. Subsp. schimperi: pulegone, menthone | Wistar albino mice (25–35 g) were subjected to second-degree burn wounds. | Essential oils were incorporated into an ointment and applied daily to mice for 21 days | The A. judaica essential oils showed antimicrobial activity against E. coli, K. pneumonide, S. aureus, B. cereus, and C. albicans. The subsp. typhoides showed better wound healing activity with skin regeneration, new skin appendages formation, and increased deposition of collagen and number of fibroblasts | Acute | [82] |
| Thymus vulgaris honey | Thymus Essential oils from O. vulgare, Rosmarinus officinalis, and Thymus vulgaris | Wistar rats (180–200 g, 3–5 months) were induced to acute dermal toxicity. Rabbits were used for wound healing test | Acute dermal irritation test: essential oils from each plant species were used at 0.5 and 5%. Wound healing assay: treatments with a mix of T. vulgaris honey and O. vulgare essential oil, mix of T. vulgaris honey and T. vulgaris essential oil, and a mix of T. vulgaris honey and R. officinalis essential oil were applied every 24 h for 5 days | The highest wound healing activity was shown for the mix of T. vulgaris honey and T. vulgaris essential oil, with wound closure rates for both thermal and chemical-induced burns of 85.21% and 82.14%, respectively | Acute | [83] |
| Rosmarinus officinalis | Essential oils of R. officinalis were incorporated into nanoencapsulated lipid nanocarriers | Mice | The animals were treated daily for 14 days with a gel containing 0.5% essential oil and a 0.5% gel with nanoencapsulated essential oils | Both R. officinalis essential oil treatments showed lower wound areas, exerted antimicrobial activity, increased levels of the anti-inflammatory cytokines IL-3, IL-10, VEGF, and SDF-1α | Acute | [84] |
5. Mechanisms of Action of the Natural Products in Wound Healing
5.1. Antioxidant
5.2. Anti-Inflammatory
5.3. Antimicrobial
6. Challenges and Solutions in the Use of Natural Products, as Wound Healing Agents
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka, T.; Kan, H.M.; Laurencin, C.T. Regenerative Engineering Approaches to Scar-Free Skin Regeneration. Regen. Eng. Transl. Med. 2022, 8, 225–247. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, H.; Ahmad, M.; Rahman, A.; Yaqeen, Z.; Sohail, T.; Fatima, N.; Iqbal, W.; Yaqeen, S.S. Evaluation of wound healing effects between Salvadora persica ointment and Solcoseryl jelly in animal model. Pak. J. Pharm. Sci. 2015, 28, 1777–1780. [Google Scholar] [PubMed]
- Kumar, B.; Vijayakumar, M.; Govindarajan, R.; Pushpangadan, P. Ethnopharmacological approaches to wound healing--exploring medicinal plants of India. J. Ethnopharmacol. 2007, 114, 103–113. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Mühlstädt, M.; Thomé, C.; Kunte, C. Rapid wound healing of scalp wounds devoid of periosteum with milling of the outer table and split-thickness skin grafting. Br. J. Dermatol. 2012, 167, 343–347. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Szycher, M.; Lee, S.J. Modern wound dressings: A systematic approach to wound healing. J. Biomater. Appl. 1992, 7, 142–213. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Falahatzadeh, M.; Raoofi, E.; Zarshenas, M.M. An Evidence-Based Review on Wound Healing Herbal Remedies From Reports of Traditional Persian Medicine. J. Evid.-Based Complement. Altern. Med. 2017, 22, 334–343. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Dhiman, A.; Nanda, S. Traditional Indian Medicinal Plants with Potential Wound Healing Activity: A Review. Int. J. Pharm. Sci. Res. 2016, 7, 1809–1819. [Google Scholar]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Hawkley, L.C. Social isolation and health, with an emphasis on underlying mechanisms. Perspect. Biol. Med. 2003, 46, S39–S52. [Google Scholar] [CrossRef]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol. Pharm. 2021, 18, 550–575. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.U.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.I.; Hussain, S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. 2015, 22, 4. [Google Scholar] [CrossRef] [Green Version]
- Ti, Y.L.; Song, F.; Fang, Z.; Zhang, P. Plants and phytochemicals inhibit scar formation: A systematic review. Ind. Crops Prod. 2022, 185, 115113. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Mehta, M.; Branford, O.A.; Rolfe, K.J. The evidence for natural therapeutics as potential anti-scarring agents in burn-related scarring. Burn. Trauma 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free. Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2022, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Kondo, T.; Ishida, Y. Molecular pathology of wound healing. Forensic Sci. Int. 2010, 203, 93–98. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; p. 423. [Google Scholar]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.M.; Sikorski, M.J.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. Part A 2014, 102, 4182–4194. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Pandith, H.; Zhang, X.; Liggett, J.; Min, K.W.; Gritsanapan, W.; Baek, S.J. Hemostatic and wound healing properties of Chromolaena odorata leaf extract. ISRN Dermatol. 2013, 2013, 168269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurahashi, T.; Fujii, J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Sinno, H.; Prakash, S. Complements and the wound healing cascade: An updated review. Plast. Surg. Int. 2013, 2013, 146764. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, M. The Tissue Factor Pathway and Wound Healing. Semin. Thromb. Hemost. 2018, 44, 142–150. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Biomechanical modeling of progressive wound healing: A computational study. Biomed. Eng. Adv. 2022, 4, 100055. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Hulkower, K.I.; Herber, R.L. Cell Migration and Invasion Assays as Tools for Drug Discovery. Pharmaceutics 2011, 3, 107–124. [Google Scholar] [CrossRef] [Green Version]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Tinpun, K.; Nakpheng, T.; Padmavathi, A.R.; Srichana, T. In Vitro Studies of Jatropha curcas L. Latex Spray Formulation for Wound Healing Applications. Turk. J. Pharm. Sci. 2020, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.J.J.; Horsten, S.F.A.J.; Kettenes-van den Bosch, J.J.; Kroes, B.H.; Beukelman, C.J.; Leeflang, B.R.; Labadie, R.P. Curcacycline A—A novel cyclic octapeptide isolated from the latex of Jatropha curcas L. FEBS Lett. 1995, 358, 215–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alilou, M.; Marzocco, S.; Hofer, D.; Rapa, S.F.; Asadpour, R.; Schwaiger, S.; Troppmair, J.; Stuppner, H. Labdane-Type Diterpenes from the Aerial Parts of Rydingia persica: Their Absolute Configurations and Protective Effects on LPS-Induced Inflammation in Keratinocytes. J. Nat. Prod. 2020, 83, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Yeh, H.-Y.; Liao, Z.-H.; Hung, S.-W.; Chen, B.; Lee, P.-T.; Nan, F.-H.; Shih, W.-L.; Chang, C.-C.; Lee, M.-C. An in vitro study shows the potential of Nostoc commune (Cyanobacteria) polysaccharides extract for wound-healing and anti-allergic use in the cosmetics industry. J. Funct. Foods 2021, 87, 104754. [Google Scholar] [CrossRef]
- Kamarazaman, I.S.; Mohamad Ali, N.A.; Abdullah, F.; Che Saad, N.; Ali, A.A.; Ramli, S.; Rojsitthisak, P.; Halim, H. In vitro wound healing evaluation, antioxidant and chemical profiling of Baeckea frutescens leaves ethanolic extract. Arab. J. Chem. 2022, 15, 103871. [Google Scholar] [CrossRef]
- Bolla, S.R.; Mohammed Al-Subaie, A.; Yousuf Al-Jindan, R.; Papayya Balakrishna, J.; Kanchi Ravi, P.; Veeraraghavan, V.P.; Arumugam Pillai, A.; Gollapalli, S.S.R.; Palpath Joseph, J.; Surapaneni, K.M. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Rodrigues, R.; Yaochite, J.N.U.; Sasahara, G.L.; Albuquerque, A.A.; da Cruz Fonseca, S.G.; de Vasconcelos Araújo, T.D.; Santiago, G.M.P.; de Sousa, L.M.; de Carvalho, J.L.; Alves, A.P.N.N.; et al. Antioxidant, anti-inflammatory and healing potential of ethyl acetate fraction of Bauhinia ungulata L. (Fabaceae) on in vitro and in vivo wound model. Mol. Biol. Rep. 2020, 47, 2845–2859. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Amen, Y.; Nagata, M.; Matsumoto, M.; Wang, D.; Shimizu, K. Anti-Phototoxicity Effect of Phenolic Compounds from Acetone Extract of Entada phaseoloides Leaves via Activation of COX-2 and iNOS in Human Epidermal Keratinocytes. Molecules 2022, 27, 440. [Google Scholar] [CrossRef]
- Elsaid, M.B.; Elnaggar, D.M.; Owis, A.I.; AbouZid, S.F.; Eldahmy, S. Production of isoquinoline alkaloids from the in vitro conserved Fumaria parviflora and their in vitro wound healing activity. Nat. Prod. Res. 2022, 36, 2575–2579. [Google Scholar] [CrossRef]
- Kaptaner İğci, B.; Aytaç, Z. An Investigation on the In Vitro Wound Healing Activity and Phytochemical Composition of Hypericum pseudolaeve N. Robson Growing in Turkey. Turk. J. Pharm. Sci. 2020, 17, 610–619. [Google Scholar] [CrossRef]
- Chiangnoon, R.; Samee, W.; Uttayarat, P.; Jittachai, W.; Ruksiriwanich, W.; Sommano, S.R.; Athikomkulchai, S.; Chittasupho, C. Phytochemical Analysis, Antioxidant, and Wound Healing Activity of Pluchea indica L. (Less) Branch Extract Nanoparticles. Molecules 2022, 27, 635. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, E.; Halim, H.; Anuar, T.S.; Hussain, R. Wound Healing Potential of Rhodomyrtus tomentosa and its Bioactive Compounds-Rhodomyrtone. J. Pharm. Res. Int. 2021, 33, 262–273. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Moridi Farimani, M.; Hamburger, M.; Jabbarzadeh, E. Wound Healing Potential of Chlorogenic Acid and Myricetin-3-O-β-Rhamnoside Isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasathkumar, M.; Raja, K.; Vasanth, K.; Khusro, A.; Sadhasivam, S.; Sahibzada, M.U.K.; Gawwad, M.R.A.; Al Farraj, D.A.; Elshikh, M.S. Phytochemical screening and in vitro antibacterial, antioxidant, anti-inflammatory, anti-diabetic, and wound healing attributes of Senna auriculata (L.) Roxb. leaves. Arab. J. Chem. 2021, 14, 103345. [Google Scholar] [CrossRef]
- Figueiredo, F.d.F.; Cechinel Filho, V.; Damazo, A.S.; Arunachalam, K.; Colodel, E.M.; Ribeiro, M.; Venturini, C.L.; Oliveira, D.M.; Machado, M.T.M.; Pavan, E.; et al. Sorocea guilleminiana Gaudich.: Wound healing activity, action mechanisms, and chemical characterization of the leaf infusion. J. Ethnopharmacol. 2020, 248, 112307. [Google Scholar] [CrossRef]
- Yen, J.-H.; Chio, W.-T.; Chuang, C.-J.; Yang, H.-L.; Huang, S.-T. Improved Wound Healing by Naringin Associated with MMP and the VEGF Pathway. Molecules 2022, 27, 1695. [Google Scholar] [CrossRef]
- Carmo, J.; Cavalcante-Araújo, P.; Silva, J.; Ferro, J.; Correia, A.C.; Lagente, V.; Barreto, E. Uvaol Improves the Functioning of Fibroblasts and Endothelial Cells and Accelerates the Healing of Cutaneous Wounds in Mice. Molecules 2020, 25, 4982. [Google Scholar] [CrossRef]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef]
- Abdelfattah, M.A.O.; Dmirieh, M.; Ben Bakrim, W.; Mouhtady, O.; Ghareeb, M.A.; Wink, M.; Sobeh, M. Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidences from in silico, in vitro and in vivo studies. J. Ethnopharmacol. 2022, 292, 115187. [Google Scholar] [CrossRef]
- Adadi, I.; Ayadi, R.E.; Bentayeb, A.; Aaziz, H.; Bouymajane, A.; Altemimi, A.B.; Cacciola, F.; Ibaoui, H.E. Phytochemical profile, in vivo anti-inflammatory and wound healing activities of the aqueous extract from aerial parts of Cistus ladanifer L. J. Pharm. Biomed. Anal. 2022, 219, 114960. [Google Scholar] [CrossRef]
- Akbari, F.; Azadbakht, M.; Bagheri, A.; Vahedi, L. In Vitro and In Vivo Wound Healing Activity of Astragalus floccosus Boiss. (Fabaceae). Adv. Pharmacol. Pharm. Sci. 2022, 2022, 7865015. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Basilio Heredia, J. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; Ezzat, S.M.; El Naggar, M.M.; El Hawary, S.S. In vivo diabetic wound healing effect and HPLC–DAD–ESI–MS/MS profiling of the methanol extracts of eight Aloe species. Rev. Bras. Farmacogn. 2016, 26, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.; Hartl, M.; Tschaikowsky, M.; Balzer, B.N.; Booth, B.W. Degradation and release of tannic acid from an injectable tissue regeneration bead matrix in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Kivanany, P.B.; Nicholas, S.E.; Nag, O.K.; Elliott, M.H.; Petroll, W.M.; Karamichos, D. Quercetin Decreases Corneal Haze In Vivo and Influences Gene Expression of TGF-Beta Mediators In Vitro. Metabolites 2022, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Ghodrati, M.; Farahpour, M.R.; Hamishehkar, H. Encapsulation of Peppermint essential oil in nanostructured lipid carriers: In-vitro antibacterial activity and accelerative effect on infected wound healing. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 161–169. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cells Nanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo wound healing and in vitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A.A. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plants Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plants Essential Oils. Antioxidants 2022, 11, 332. [Google Scholar] [CrossRef]
- Haikal, A.; El-Neketi, M.; Awadin, W.F.; Hassan, M.A.; Gohar, A.A. Essential oils from wild Mentha longifolia subspecies typhoides and subspecies schimperi: Burn wound healing and antimicrobial candidates. J. King Saud Univ.-Sci. 2022, 34, 102356. [Google Scholar] [CrossRef]
- Mekkaoui, M.; Assaggaf, H.; Qasem, A.; El-Shemi, A.; Abdallah, E.M.; Bouidida, E.H.; Naceiri Mrabti, H.; Cherrah, Y.; Alaoui, K. Ethnopharmacological Survey and Comparative Study of the Healing Activity of Moroccan Thyme Honey and Its Mixture with Selected Essential Oils on Two Types of Wounds on Albino Rabbits. Foods 2022, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 980–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plants: Rockville, MD, USA, 2015; pp. 1250–1318. [Google Scholar]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, F1000 Faculty Rev-787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, A.J. Healing Mechanisms in Cutaneous Wounds: Tipping the Balance. Tissue Eng. Part B Rev. 2022, 28, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Cardona, A.F.; Wilson, S.E. Skin and soft-tissue infections: A critical review and the role of telavancin in their treatment. Clin. Infect. Dis. 2015, 61 (Suppl. 2), S69–S78. [Google Scholar] [CrossRef] [Green Version]
- Andonova, M.; Urumova, V. Immune surveillance mechanisms of the skin against the stealth infection strategy of Pseudomonas aeruginosa-review. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 433–448. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, A.A.; Sani, D.; Suleiman, M.M.; Ahmad, A.; Hassan, A.Z. Assessment of antioxidant and wound healing activity of the crude methanolic extract of Diospyros mespiliformis Hochst. ex A. DC. (Ebenaceae) and its fractions in Wistar rats. S. Afr. J. Bot. 2022, 150, 305–312. [Google Scholar] [CrossRef]
- Agour, A.; Mssillou, I.; Es-safi, I.; Conte, R.; Mechchate, H.; Slighoua, M.; Amrati, F.E.-Z.; Parvez, M.K.; Numan, O.; Bari, A.; et al. The Antioxidant, Analgesic, Anti-Inflammatory, and Wound Healing Activities of Haplophyllum tuberculatum (Forsskal) A. Juss Aqueous and Ethanolic Extract. Life 2022, 12, 1553. [Google Scholar] [CrossRef]
- Espinosa-Espinosa, L.; Garduño-Siciliano, L.; Rodriguez-Canales, M.; Hernandez-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The Wound-Healing Effect of Mango Peel Extract on Incision Wounds in a Murine Model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef]
- Rocha, M.I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Pereira, C.; Moreira, P.; Salgueiro, L.; Figueirinha, A. Chemical characterization and bioactive potential of Artemisia campestris L. subsp. maritima (DC) Arcang. essential oil and hydrodistillation residual water. J. Ethnopharmacol. 2021, 276, 114146. [Google Scholar] [CrossRef]
- Shady, N.H.; Soltane, R.; Maher, S.A.; Saber, E.A.; Elrehany, M.A.; Mostafa, Y.A.; Sayed, A.M.; Abdelmohsen, U.R. Wound Healing and Antioxidant Capabilities of Zizyphus mauritiana Fruits: In-Vitro, In-Vivo, and Molecular Modeling Study. Plants 2022, 11, 1392. [Google Scholar] [CrossRef]
- Murthy, S.; Gautam, M.; Goel, S.; Purohit, V.; Sharma, H.; Goel, R. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. BioMed Res. Int. 2013, 2013, 972028. [Google Scholar] [CrossRef] [Green Version]
- Estevão, L.R.M.; Simões, R.S.; Cassini-Vieira, P.; Canesso, M.C.C.; Barcelos, L.D.S.; Rachid, M.A.; Câmara, C.; Evêncio-Neto, J. Schinus terebinthifolius Raddi (Aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice 1. Acta Cir. Bras. 2017, 32, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Vennila, V.; Udayakumar, R. Wound Healing Efficacy of Herbal Preparations of Croton bonplandianum Baill. on Excision Wound in Experimental Rats. Curr. Tradit. Med. 2017, 3, 38–50. [Google Scholar] [CrossRef]
- Lei, Z.; Cao, Z.; Yang, Z.; Ao, M.; Jin, W.; Yu, L. Rosehip Oil Promotes Excisional Wound Healing by Accelerating the Phenotypic Transition of Macrophages. Planta Med. 2019, 85, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Scagnelli, A.M. Therapeutic Review: Manuka Honey. J. Exot. Pet Med. 2016, 25, 168–171. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.T.; Ke, C.Y.; Wu, W.T.; Harn, H.J.; Tseng, Y.H.; Lee, R.P. Effects of Angelica dahurica and Rheum officinale Extracts on Excisional Wound Healing in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 1583031. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.H.; Choi, S.; Kim, B.H. Skin Wound Healing Effects and Action Mechanism of Acai Berry Water Extracts. Toxicol. Res. 2017, 33, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levet, A.; Bordes, C.; Clément, Y.; Mignon, P.; Morell, C.; Chermette, H.; Marote, P.; Lantéri, P. Acute aquatic toxicity of organic solvents modeled by QSARs. J. Mol. Model. 2016, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.S.; Clary, J.J.; Terrill, J.B.; Bolte, H.F. Subchronic inhalation toxicity of methanol. J. Toxicol. Environ. Health 1987, 20, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Morais, F.P.; Curto, J.M.R. Design and Engineering of Natural Cellulose Fiber-Based Biomaterials with Eucalyptus Essential Oil Retention to Replace Non-Biodegradable Delivery Systems. Polymers 2022, 14, 3621. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Ahmad, L.; He, Y.; Hao, J.-C.; Semotiuk, A.; Liu, Q.-R.; Mazari, P. Toxic pyrrolizidine alkaloids provide a warning sign to overuse of the ethnomedicine Arnebia benthamii. J. Ethnopharmacol. 2018, 210, 88–94. [Google Scholar] [CrossRef]
- Yang, M.; Ma, J.; Ruan, J.; Ye, Y.; Fu, P.P.-C.; Lin, G. Intestinal and hepatic biotransformation of pyrrolizidine alkaloid N-oxides to toxic pyrrolizidine alkaloids. Arch. Toxicol. 2019, 93, 2197–2209. [Google Scholar] [CrossRef]
- Agus, H.H. Chapter 4—Terpene toxicity and oxidative stress. In Toxicology; Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 33–42. [Google Scholar]
- Karlberg, A.-T.; Lepoittevin, J.-P. One hundred years of allergic contact dermatitis due to oxidized terpenes: What we can learn from old research on turpentine allergy. Contact Dermat. 2021, 85, 627–636. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Convers. Biorefinery 2022, 12, 2949–2962. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and delivery of bioactive citrus pomace polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Sustainable biomaterials and their applications: A short review. Mater. Today Proc. 2020, 30, 274–282. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Silkstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]




| Source (Type of Extract) | Main Components | Cell Line/Dose * | Key Results | Reference |
|---|---|---|---|---|
| Aristolochia saccata leaves (methanolic extract) | NE | Mouse fibroblasts (L929)/250 µg/mL, 48 h | ↑Cell migration ↑Collagen-1 expression | [57] |
| Bahuinia ungulate stem wood (ethyl acetate fraction) | Phenolic compounds, tannins, fisetinidol (flavanol) | Human epithelial (A549)/10 and 100 µL, 24 h | ↑Cell migration | [58] |
| Entada phaseoloides leaves (acetone extract) | Protocatechuic acid, epicatechin, kaempferol | Human keratinocyte (HaCat)/100 µM | ↑Cell migration during wound closure | [59] |
| Fumaria parviflora aerial parts (ethanolic extract) | Sanguinarine (isoquinoline alkaloid) | Human skin fibroblasts/1 µg/mL, 24 h | ↑Cell migration ↑Wound closure percentage | [60] |
| Hypericum pseudolaeve aerial parts (methanolic and aqueous extracts) | Gallic acid, catechin, chlorogenic acid, syringic acid, epicatechin, rutin, quercitrin, and quercetin, among other phenolic compounds | L929/62 µg/mL, 24 h | ↑Wound closure percentage | [61] |
| luchea indica branches (ethanolic extract) | Flavonoids, phenolic compounds, tannins, alkaloids, and terpenoids | Primary epidermal keratinocytes/62.5 µg/mL, 2 h Human dermal fibroblasts/62.5 and 125 µg/mL, 2 h Oral mucosal keratinocytes (HO-1-N-1)/62.5 and 125 µg/mL, 2 h | ↓Cell gap area | [62] |
| Rhodomyrtus tormentosa leaves (ethanolic extract) | Saponins, flavonoids, steroids, tannins, phenolic compounds | Human fibroblasts/62.5 µg/mL, 24 h | ↑Rate of cell migration | [63] |
| Parrotia persica | Myricetin-3-O-β-rhamnoside, chlorogenic acid | Normal human keratinocyte (NHEK), normal human dermal fibroblast (NHDF)/ 10 µg/mL, 24 h Human umbilical vein endothelial (HUVEC)/10 µg/mL, 8 h | ↑Wound closure percentage (NHEK, NHDF) ↑Number of tubular network formation (HUVEC) | [64] |
| Senna auriculata leaves (methanolic extract) | Mome inositol, 13-docosenamide, (Z)-, cycloheptasiloxane, tetradecamethyl-, and octadecanoic acid, 2-hydroxy-1- (hydroxymethyl)ethyl ester, alkaloids, flavonoids, phenolic compounds, and tannins | L929/25 and 50 µg/mL, 24 h | ↑Rate of wound healing | [65] |
| Sorocea guilleminiana leaves (infusion) | Salicylic acid, gallic acid, pinocembrin, and isoquercitrin, among other phenolic compounds | Mouse embryo fibroblast (NIH-3T3)/ 4 µg/mL, 18 h | ↑Cell migration/proliferation | [66] |
| Standard | Naringenin (flavanone) | HaCat/200 µM, 24 h | ↑MMP-2, MMP-9, MMP-14, VEGF-A expression and ↑Cell migration | [67] |
| Standard | Uvaol (terpene) | NIH-3T3 and endothelioma tEnd.1/50 µM, 24 h | ↑Cell motility and migration ↑Fibronectin and laminin deposition in fibroblasts | [68] |
| Standard | Vitexin (glycosyl-flavone) | NIH-3T3 and HaCat/1 µg/well, 72 h | ↑Cell migration | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. https://doi.org/10.3390/molecules28020598
Criollo-Mendoza MS, Contreras-Angulo LA, Leyva-López N, Gutiérrez-Grijalva EP, Jiménez-Ortega LA, Heredia JB. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules. 2023; 28(2):598. https://doi.org/10.3390/molecules28020598
Chicago/Turabian StyleCriollo-Mendoza, Marilyn S., Laura A. Contreras-Angulo, Nayely Leyva-López, Erick P. Gutiérrez-Grijalva, Luis Alfonso Jiménez-Ortega, and J. Basilio Heredia. 2023. "Wound Healing Properties of Natural Products: Mechanisms of Action" Molecules 28, no. 2: 598. https://doi.org/10.3390/molecules28020598
APA StyleCriollo-Mendoza, M. S., Contreras-Angulo, L. A., Leyva-López, N., Gutiérrez-Grijalva, E. P., Jiménez-Ortega, L. A., & Heredia, J. B. (2023). Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules, 28(2), 598. https://doi.org/10.3390/molecules28020598