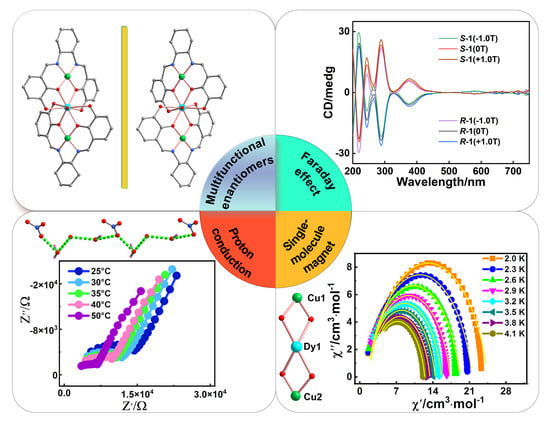
A Pair of Multifunctional Cu(II)–Dy(III) Enantiomers with Zero–Field Single–Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. X–ray Single–Crystal Structure Determination
2.2. Crystal Structural Descriptions of R–1
2.3. Thermal Stability
2.4. Circular Dichroism (CD) and Magnetic Circular Dichroism (MCD)
2.5. Magnetic Properties
2.6. Proton Conduction
3. Experimental Section
3.1. Materials and Instruments
3.2. Syntheses of R–1 and S–1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rikken, J.L.J.A.; Raupach, E. Observation of magneto–chiral dichroism. Nature 1997, 390, 493–494. [Google Scholar] [CrossRef]
- Barron, L.D. Chirality, magnetism and light. Nature 2000, 405, 895–896. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Liu, C.M.; Xiong, R.G.; Zhang, D.Q.; Zhu, D.B. Nanoscale Homochiral C3–Symmetric Mixed–Valence Manganese Cluster Complexes with Both Ferromagnetic and Ferroelectric Properties. J. Am. Chem. Soc. 2010, 132, 4044–4045. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, W.C.; Li, Z.J.; Zhang, J.F.; Zhao, W.J.; Feng, Y.; Tang, B.Z.; Lee, C.S. Manipulation of Molecular Aggregation States to Realize Polymorphism, AIE, MCL, and TADF in a Single Molecule. Angew. Chem. Int. Ed. 2018, 57, 12473–12477. [Google Scholar] [CrossRef]
- Train, C.R.; Gheorghe, R.; Krstic, V.; Chamoreau, L.; Ovnesyan, N.S.; Rikken, G.L.J.A.; Gruselle, M.; Verdaguer, M. Strong magneto–chiral dichroism in enantiopure chiral ferromagnets. Nat. Mater. 2008, 7, 729–734. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ge, J.Y.; Hua, C.; Jiao, C.Q.; Wu, Y.; Leong, C.F.; D’Alessandro, D.M.; Liu, T.; Zuo, J.L. Photo– and Electronically Switchable Spin–Crossover Iron(II) Metal–Organic Frameworks Based on a Tetrathiafulvalene Ligan. Angew. Chem. Int. Ed. 2017, 56, 5465–5470. [Google Scholar] [CrossRef]
- Ou Yang, J.; Crassous, J. Chiral multifunctional molecules based on organometallic helicenes: Recent advances. Coord. Chem. Rev. 2018, 376, 533–547. [Google Scholar] [CrossRef]
- Yang, J.G.; Li, K.; Wang, J.; Sun, S.S.; Chi, W.J.; Wang, C.; Chang, X.Y.; Zou, C.; To, W.P.; Li, M.D.; et al. Controlling Metallophilic Interactions in Chiral Au(I) Double Salts towards Excitation Wavelength–Tunable Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2020, 59, 6915–6922. [Google Scholar] [CrossRef]
- Long, J.; Ivanov, M.S.; Khomchenko, V.A.; Mamontova, E.; Thibaud, J.M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.S.; Carlos, L.D.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef]
- Atzori, M.; Breslavetz, I.; Paillot, K.; Inoue, K.; Rikken, G.L.J.A.; Train, C. A Chiral, Prussian Blue Analogue Pushes Magneto–Chiral Dichroism Limits. J. Am. Chem. Soc. 2019, 141, 20022–20025. [Google Scholar] [CrossRef]
- Atzori, M.; Santanni, F.; Breslavetz, I.; Paillot, K.; Caneschi, A.; Rikken, G.L.J.A.; Sessoli, R.; Train, C. Magnetic Anisotropy Drives Magnetochiral Dichroism in a Chiral Molecular Helix Probed with Visible Light. J. Am. Chem. Soc. 2020, 142, 13908–13916. [Google Scholar] [CrossRef]
- Liu, C.M.; Sun, R.; Wang, B.W.; Wu, F.; Hao, X.; Shen, Z. Homochiral Ferromagnetic Coupling Dy2 Single–Molecule Magnetswith Strong Magneto–Optical Faraday Effects at Room Temperature. Inorg. Chem. 2021, 60, 12039–12048. [Google Scholar] [CrossRef]
- Lim, D.W.; Kitagawa, H. Proton Transport in Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef]
- Ye, Y.X.; Gong, L.S.; Xiang, S.C.; Zhang, Z.J.; Chen, B.L. Metal–Organic Frameworks as a Versatile Platform for Proton Conductors. Adv. Mater. 2020, 32, 1907090. [Google Scholar] [CrossRef]
- Han, B.X.; Jiang, Y.F.; Sun, X.R.; Li, Z.F.; Li, G. Proton conductive N–heterocyclic metal–organic frameworks. Coord. Chem. Rev. 2021, 432, 213754. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Ōkawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of Low–Humidity Proton Conduction by Controlling Hydrophilicity in Layered Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5745. [Google Scholar] [CrossRef]
- Okawa, H.; Sadakiyo, M.; Yamada, T.; Maesato, M.; Ohba, M.; Kitagawa, H. Proton–Conductive Magnetic Metal–Organic Frameworks, {NR3(CH2COOH)}[MaIIMbIII(ox)3]: Effect of Carboxyl Residue upon Proton Conduction. J. Am. Chem. Soc. 2013, 135, 2256–2262. [Google Scholar] [CrossRef]
- Pardo, E.; Train, C.; Gontard, G.; Boubekeur, K.; Fabelo, O.; Liu, H.B.; Dkhil, B.; Lloret, F.; Nakagawa, K.; Tokoro, H.; et al. High Proton Conduction in a Chiral Ferromagnetic Metal–Organic Quartz–like Framework. J. Am. Chem. Soc. 2011, 133, 15328–15331. [Google Scholar] [CrossRef]
- Bill, E. Single–molecule magnets: Iron lines up. Nat. Chem. 2013, 5, 556–557. [Google Scholar] [CrossRef]
- Katie, R.M.; Stefan, G.M.; Wayne, W.L.J.; Stosh, A.K.; David, K.S.; Tolek, T.; Jeffrey, R.L. Influence of Pyrazolate vs N-Heterocyclic Carbene Ligands on the Slow Magnetic Relaxation of Homoleptic Trischelate Lanthanide(III) and Uranium(III) Complexes. J. Am. Chem. Soc. 2014, 16, 6056–6068. [Google Scholar]
- Abbas, P.; Quinn, K.; Alexandropoulos, D.I.; Damjanović, M.; Wernsdorfer, W.; Escuer, A.; Mayans, J.; Orcid, M.P.; Stamatatos, T.C. Transition Metal Single–Molecule Magnets: A {Mn31} Nanosized Cluster with a Large Energy Barrier of ~60 K and Magnetic Hysteresis at ~5 K. J. Am. Chem. Soc. 2017, 139, 15644–15647. [Google Scholar] [CrossRef]
- Chakarawet, K.; Bunting, P.C.; Long, J.R. Large Anisotropy Barrier in a Tetranuclear Single–Molecule Magnet Featuring Low–Coordinate Cobalt Centers. J. Am. Chem. Soc. 2018, 140, 2058–2061. [Google Scholar] [CrossRef]
- Ibrahim, M.; Lan, D.Y.H.; Bassil, B.S.; Xiang, Y.X.; Suchopar, A.; Powell, A.K.; Kortz, U. Hexadecacobalt(II)–Containing Polyoxometalate–Based Single–Molecule Magnet. Angew. Chem. Int. Ed. 2011, 50, 4708–4711. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A Tetranuclear 3d−4f Single Molecule Magnet:[CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef]
- Sheikh, J.A.; Jena, H.S.; Konar, S. Co3Gd4 Cage as Magnetic Refrigerant and Co3Dy3 Cage Showing Slow Relaxation of Magnetisation. Molecules 2022, 27, 1130. [Google Scholar] [CrossRef]
- Meng, Y.S.; Xu, L.; Xiong, J.; Yuan, Q.; Liu, T.; Wang, B.W.; Gao, S. Low–Coordinate Single–Ion Magnets by Intercalation of Lanthanides into a Phenol Matrix. Angew. Chem. Int. Ed. 2018, 57, 4673–4676. [Google Scholar] [CrossRef]
- Benmansour, S.; Hernández–Paredes, A.; Bayona–Andrés, M.; Gómez–García, C.J. Slow Relaxation of the Magnetization in Anilato–Based Dy(III) 2D Lattices. Molecules 2021, 26, 1190. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, J.L.; Ungur, L.; Liu, J.; Li, Q.W.; Wang, L.F.; Ni, Z.P.; Chibotaru, L.F.; Chen, X.M.; Tong, M.L. Symmetry–Supported Magnetic Blocking at 20 K in Pentagonal Bipyramidal Dy(III) Single–Ion Magnets. J. Am. Chem. Soc. 2016, 138, 2829–2837. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ma, Y.; Chai, Y.; Shi, W.; Sun, Y.; Cheng, P. Observation of Magnetodielectric Effect in a Dysprosium–Based Single–Molecule Magnet. J. Am. Chem. Soc. 2018, 140, 7795–7798. [Google Scholar] [CrossRef]
- Kazin, P.E.; Zykin, M.A.; Utochnikova, V.V.; Magdysyuk, O.V.; Vasiliev, A.V.; Zubavichus, Y.V.; Schnelle, W.; Felser, C.; Jansen, M. “Isolated” DyO+ Embedded in a Ceramic Apatite Matrix Featuring Single–Molecule Magnet Behavior with a High Energy Barrier for Magnetization Relaxation. Angew. Chem. Int. Ed. 2017, 56, 13416–13420. [Google Scholar] [CrossRef]
- Harriman, K.L.M.; Brosmer, J.L.; Ungur, L.; Diaconescu, P.L.; Murugesu, M. Pursuit of Record Breaking Energy Barriers: A Study of Magnetic Axiality in Diamide Ligated DyIII Single–Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 1420–1423. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single–molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.C.; Liu, J.L.; Vieru, V.; Ungur, L.; Jia, J.H.; Chibotaru, L.F.; Lan, Y.H.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal–Bipyramidal Dy(III) Single–Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.S.; Jiang, S.D.; Wang, B.W.; Gao, S. Understanding the Magnetic Anisotropy toward Single–Ion Magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef]
- Ding, Y.S.; Chilton, N.F.; Winpenny, R.; Zheng, Y.Z. On Approaching the Limit of Molecular Magnetic Anisotropy: A Near–Perfect Pentagonal Bipyramidal Dysprosium(III) Single–Molecule Magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.N.; Tang, J.K. Recent advances in dysprosium–based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining new light on multifunctional lanthanide single–molecule magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- El Rez, B.; Liu, J.W.; Bereau, V.; Duhayon, C.; Horino, Y.; Suzuki, T.; Coolen, L.; Sutter, J.P. Concomitant emergence of circularly polarized luminescence and single–molecule magnet behavior in chiral–at–metal Dy complex. Inorg. Chem. Front. 2020, 7, 4527–4534. [Google Scholar] [CrossRef]
- Sato, T.; Breedlove, B.K.; Yamashita, M.; Katoh, K. Electro–Conductive Single–Molecule Magnet Composed of a Dysprosium(III)–Phthalocyaninato Double–Decker Complex with Magnetoresistance. Angew. Chem. Int. Ed. 2021, 60, 21179–21183. [Google Scholar] [CrossRef]
- Shen, Y.B.; Cosquer, G.; Zhang, H.T.; Breedlove, B.K.; Cui, M.X.; Yamashita, M. 4f–π Molecular Hybrid Exhibiting Rich Conductive Phases and Slow Relaxation of Magnetization. J. Am. Chem. Soc. 2021, 143, 9543–9550. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Zhao, C.; Feng, T.T.; Liu, X.D.; Ying, X.; Li, X.L.; Zhang, Y.Q.; Tang, J.K. Air–Stable Chiral Single–Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Qin, L.; Yu, Y.Z.; Liao, P.Q.; Xue, W.; Zheng, Z.P.; Chen, X.M.; Zheng, Y.Z.A. “Molecular Water Pipe”: A Giant Tubular Cluster {Dy72} Exhibits Fast Proton Transport and Slow Magnetic Relaxation. Adv. Mater. 2016, 28, 10772–10779. [Google Scholar] [CrossRef]
- Rouquette, J.; Jerome, L.; Marc, T.J.; Rute, F.A.S.; Bruno, D.; Luis, C.D.; Veaceslav, V.; Liviu, C.F.; Leszek, K.; Julien, H.; et al. A High–Temperature Molecular Ferroelectric Zn/Dy Complex Exhibiting Single–Ion–Magnet Behavior and Lanthanide Luminescence. Angew. Chem. Int. Ed. 2015, 127, 2264–2268. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhu, S.D.; Lu, Y.B.; Hao, X.; Wen, H.R. Homochiral Cu6Dy3 single–molecule magnets displaying proton conduction and a strong magneto–optical Faraday effect. Inorg. Chem. Front. 2023, 10, 3714–3722. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.Q.; Hao, X.; Zhu, D.B. Assembly of chiral 3d–4f wheel–like cluster complexes with achiral ligands: Single–molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Kotrle, K.; Nemec, I.; Moncol, J.; Čižmár, E.; Herchel, R. 3d–4f magnetic exchange interactions and anisotropy in a series of heterobimetallic vanadium(IV)–lanthanide(III) Schiff base complexes. Dalton Trans. 2021, 50, 13883–13893. [Google Scholar] [CrossRef]
- Bhanja, A.; Schulze, M.; Herchel, R.; Pineda, E.M.; Wernsdorfer, W.; Ray, D. Selective Coordination of Self–Assembled Hexanuclear [Ni4Ln2] and [Ni2Mn2Ln2] (Ln = DyIII, TbIII, and HoIII) Complexes: Stepwise Synthesis, Structures, and Magnetic Properties. Inorg. Chem. 2020, 59, 17929–17944. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- CrystalClear. Software User’s Guide for the Rigaku R–Axis, and Mercury and Jupiter CCD Automated X−ray Imaging System; Version 1.35; Rigaku Molecular Structure Corporation: Akishima, Japan, 2008. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; Electronic Structure Group, Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Litvinova, Y.M.; Gayfulin, Y.M.; Leusen, J.; Samsonenko, D.G.; Lazarenko, V.A.; Zubavichus, Y.V.; Kogerler, P.; Mironov, Y.V. Metal–organic frameworks based on polynuclear lanthanide complexes and octahedral rhenium clusters. Inorg. Chem. Front. 2019, 6, 1518–1526. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Mart, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single–Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef]
- Jabeur, W.; Msalmi, R.; Korb, M.; Mosconi, E.; Tozri, A.; Althubiti, N.A.; Naili, H. Optical and magnetic characterization of one–dimensional Cu(II)–based perovskite: A high UV–Vis–NIR absorber. J. Mater. Chem. C 2021, 9, 17158–17166. [Google Scholar] [CrossRef]
- Portillo Moreno, O.; Portillo Araiza, O.R.; Chavez Portillo, M.; Carranza Tellez, V.; Vicencio Garrido, M.A. Jahn–Teller effect analysis at coordination complex [Cu(NH3)4]2+ ion, growth by green synthesis in CuS nanocrystals. Optik 2022, 251, 168470. [Google Scholar] [CrossRef]
- Wen, H.R.; Bao, J.; Liu, S.J.; Liu, C.M.; Zhang, C.W.; Tang, Y.Z. Temperature–controlled polymorphism of chiral CuII–LnIII dinuclear complexes exhibiting slow magnetic relaxation. Dalton Trans. 2015, 44, 11191–11201. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wu, G.; Xu, H.; Wang, X.; Long, L.S.; Kong, X.J.; Zheng, L.S. Magnetooptical Properties of Chiral [Co2Ln] Clusters. Inorg. Chem. 2020, 59, 193–197. [Google Scholar] [CrossRef]
- Cai, G.; Bozhkova, N.; Odingo, J.; Berova, N.; Nakanishi, K. Circular dichroism exciton chirality method. New red–shifted chromophores for hydroxyl groups. J. Am. Chem. Soc. 1993, 115, 7192–7198. [Google Scholar] [CrossRef]
- Seo, M.S.; Sun, D.; Kim, H. Stereoselective Chiral Recognition of Amino Alcohols with 2,2′–Dihydroxybenzil. J. Org. Chem. 2017, 82, 6586–6591. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, S.; Wang, H.; Dou, J.; Jiang, J. Magneto–chiral dichroism in chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple–decker SMMs. Inorg. Chem. Front. 2014, 1, 167–171. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Segawa, H.; Ishii, K. Magneto–Chiral Dichroism of Organic Compounds. Angew. Chem. Int. Ed. 2011, 50, 9133–9136. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Wada, S.; Yanagisawa, K.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. Molecular Design Guidelines for Large Magnetic Circular Dichroism Intensities in Lanthanide Complexes. ChemPhysChem 2016, 17, 845–849. [Google Scholar] [CrossRef]
- Huang, X.D.; Xu, Y.; Fan, K.; Bao, S.S.; Kurmoo, M.; Zheng, L.M. Reversible SC–SC Transformation involving [4 + 4] Cycloaddition of Anthracene: A Single–Ion to Single–Molecule Magnet and Yellow–Green to Blue–White Emission. Angew. Chem. Int. Ed. 2018, 57, 8577–8581. [Google Scholar] [CrossRef] [PubMed]
- Latendresse, T.P.; Bhuvanesh, N.S.; Nippe, M. Hard Single–Molecule Magnet Behavior by a Linear Trinuclear Lanthanide–[1]Metallocenophane Complex. J. Am. Chem. Soc. 2017, 139, 14877–14880. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.Q.; Zhu, D.B. Fine Tuning the Energy Barrier of Molecular Nanomagnets via Lattice Solvent Molecules. Sci. Rep. 2017, 7, 15483. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.L.; Zou, H.H.; Zhang, Z.; Sun, W.Y.; Liang, F.P. Two types of Cu–Ln Heterometallic Coordination Polymers with 2–Hydroxyisophthalate: Syntheses, Structures and Magnetic Properties. Cryst. Growth Des. 2015, 15, 2883–2890. [Google Scholar] [CrossRef]
- Fatila, E.M.; Rouzieres, M.; Jennings, M.C.; Lough, A.J.; Clerac, R.; Preuss, K.E. Fine–Tuning the Single–Molecule Magnet Properties of a [Dy(III)–Radical](2) Pair. J. Am. Chem. Soc. 2013, 135, 9596–9599. [Google Scholar] [CrossRef]
- Ma, Y.J.; Hu, J.X.; Han, S.D.; Li, J.H.; Wang, G.M. Manipulating On/Off Single–Molecule Magnet Behavior in a Dy(III)–Based Photochromic Complex. J. Am. Chem. Soc. 2020, 142, 2682–2689. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Wang, K.; Xi, L.; Ma, Y.; Li, L.C. Slow relaxation of magnetization in unprecedented Cu–Ln–Rad hetero–tri–spin chains constructed from multidentate nitronyl nitroxide. J. Mater. Chem. C 2019, 7, 9057–9064. [Google Scholar] [CrossRef]
- Ling, B.K.; Zhai, Y.Q.; Jin, P.B.; Ding, H.F.; Zhang, X.F.; Lv, Y.; Fu, Z.D.; Deng, J.W.; Schulze, M.; Wernsdorfer, W.; et al. Suppression of zero–field quantum tunneling of magnetization by a fluorido bridge for a “very hard” 3d–4f single–molecule magnet. Matter 2022, 5, 3485–3498. [Google Scholar] [CrossRef]
- Yao, Z.Z.; Pan, L.; Liu, L.Z.; Zhang, J.D.; Lin, Q.J.; Ye, Y.X.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Simultaneous implementation of resistive switching and rectifying effects in a metal–organic framework with switched hydrogen bond pathway. Sci. Adv. 2019, 5, eaaw4515. [Google Scholar] [CrossRef]
- Afzal, J.; Fu, Y.; Luan, T.X.; Su, Z.; Li, P.Z. Highly Effective Proton–Conduction Matrix–Mixed Membrane Derived from an –SO3H Functionalized Polyamide. Molecules 2022, 27, 4110. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Hu, J.-J.; Dong, L.; Wen, H.-R.; Liu, S.-J.; Lu, Y.-B.; Liu, C.-M. Multifunctional Zn(II)–Yb(III) complex enantiomers showing second–harmonic generation, near–infrared luminescence, single–molecule magnet behaviour and proton conduction. J. Mater. Chem. C 2020, 8, 16032–16041. [Google Scholar] [CrossRef]
- Kallem, P.; Drobek, M.; Julbe, A.; Vriezekolk, E.J.; Mallada, R.; Pina, M.P. Hierarchical Porous Polybenzimidazole Microsieves: An Efficient Architecture for Anhydrous Proton Transport via Polyionic Liquids. ACS Appl. Mater. Interfaces 2017, 9, 14844–14857. [Google Scholar] [CrossRef]
- Umeyama, D.; Horike, S.; Inukai, M.; Takura, T.; Kitagawa, S. Inherent Proton Conduction in a 2D Coordination Framework. J. Am. Chem. Soc. 2012, 134, 12780–12785. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Zheng, S.-L.; Wu, C.-M.; Ye, X.-H.; Liao, W.-M.; He, J. Structure, Luminescent Sensing and Proton Conduction of a Boiling–Water–Stable Zn(II) Metal–Organic Framework. Molecules 2021, 26, 5044. [Google Scholar] [CrossRef]
- Kim, S.; Joarder, B.; Hurd, J.; Zhang, J.; Dawson, K.W.; Gelfand, B.S.; Wong, N.E.; Shimizu, G.K.H. Achieving Superprotonic Conduction in Metal–Organic Frameworks through Iterative Design Advances. J. Am. Chem. Soc. 2018, 140, 1077–1082. [Google Scholar] [CrossRef]
- Zhang, F.M.; Dong, L.Z.; Qin, J.S.; Guan, W.; Liu, J.; Li, S.L.; Lu, M.; Lan, Y.Q.; Su, Z.M.; Zhou, H.C. Effect of Imidazole Arrangements on Proton–Conductivity in Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef]
- Lassoued, M.S.; Bi, L.Y.; Wu, Z.X.; Zhou, G.J.; Zheng, Y.Z. Piperidine–induced Switching of the direct band gaps of Ag(I)/Bi(III) bimetallic iodide double perovskites. J. Mater. Chem. C 2020, 8, 5349–5354. [Google Scholar] [CrossRef]
- Zhu, M.; Hao, Z.M.; Song, X.Z.; Meng, X.; Zhao, S.N.; Song, S.Y.; Zhang, H.J. A New Type of Double–Chain Based 3D Lanthanide(III) Metal–Organic Framework Demonstrating Proton Conduction and Tunable Emission. Chem. Commun. 2014, 50, 1912–1914. [Google Scholar] [CrossRef]
- Wong, N.E.; Ramaswamy, P.; Lee, A.S.; Gelfand, B.S.; Bladek, K.J.; Taylor, J.M.; Spasyuk, D.M.; Shimizu, G.K.H. Tuning Intrinsic and Extrinsic Proton Conduction in Metal–Organic Frameworks by the Lanthanide Contraction. J. Am. Chem. Soc. 2017, 139, 14676–14683. [Google Scholar] [CrossRef]
- Li, S.J.; Zhao, Y.; Knoll, S.; Liu, R.J.; Li, G.; Peng, Q.P.; Qiu, P.T.; He, D.F.; Streb, C.; Chen, X.N. High Proton–Conductivity in Covalently Linked Polyoxometalate–Organoboronic Acid–Polymers. Angew. Chem. Int. Ed. 2021, 60, 16953–16957. [Google Scholar] [CrossRef]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau–Corcos, C.; Hamdane, D.; Maurin, G.; Devautour–Vinot, S.; et al. A Robust Zirconium Amino Acid Metal–Organic Framework for Proton Conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Aonuma, T.; Tamaki, T.; Ohashi, H.; Ushiyama, H.; Yamashitac, K.; Yamaguchi, T. The proton conduction mechanism in a material consisting of packed acids. Chem. Sci. 2014, 5, 4878–4887. [Google Scholar] [CrossRef]
- Qin, Y.; Gao, T.L.; Xie, W.P.; Li, Z.F.; Li, G. Ultrahigh Proton Conduction in Two Highly Stable Ferrocenyl Carboxylate Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 31018–31027. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Huang, J.; Liao, Y.-Q.; Lin, X.-L.; Huang, S.-Y.; Liu, C.-M.; Wen, H.-R.; Liu, S.-J.; Wang, F.-Y.; Zhu, S.-D. Multifunctional Dinuclear Dy–Based Coordination Complex Showing Visible Photoluminescence, Single–Molecule Magnet Behavior, and Proton Conduction. Inorg. Chem. 2022, 61, 18545–18553. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Dong, L.; Hu, J.-J.; Wen, H.-R.; Lu, Y.-B.; Deng, W.-H.; Liu, C.-M.; Liu, S.-J.; Xu, G.; Fu, Z.-H. A proton conductor showing an indication of single–ion magnet behavior based on a mononuclear Dy(iii) complex. J. Mater. Chem. C 2021, 9, 481–488. [Google Scholar] [CrossRef]
- Liang, X.Q.; Zhang, F.; Feng, W.; Zou, X.Q.; Zhao, C.J.; Na, H.; Liu, C.; Sun, F.X.; Zhu, G.S. From metal–organic framework (MOF) to MOF–polymer composite membrane: Enhancement of low–humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Pili, S.; Argent, S.P.; Morris, C.G.; Rought, P.; Garcia–Sakai, V.; Silverwood, I.P.; Easun, T.L.; Li, M.; Warren, M.R.; Murray, C.A.; et al. Proton Conduction in a Phosphonate–Based Metal–Organic Framework Mediated by Intrinsic “Free Diffusion inside a Sphere”. J. Am. Chem. Soc. 2016, 138, 6352–6355. [Google Scholar] [CrossRef]
- Markus, A.; Ingo, J.; Susanne, K.; Patrick, W.; Birgit, W.; Roland, F. Dicatechol–diimines: Easily accessible ligands for the self–assembly of dinuclear triple–stranded helicates. Dalton Trans. 2004, 1, 37–43. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-D.; Zhou, Y.-L.; Liu, F.; Lei, Y.; Liu, S.-J.; Wen, H.-R.; Shi, B.; Zhang, S.-Y.; Liu, C.-M.; Lu, Y.-B. A Pair of Multifunctional Cu(II)–Dy(III) Enantiomers with Zero–Field Single–Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects. Molecules 2023, 28, 7506. https://doi.org/10.3390/molecules28227506
Zhu S-D, Zhou Y-L, Liu F, Lei Y, Liu S-J, Wen H-R, Shi B, Zhang S-Y, Liu C-M, Lu Y-B. A Pair of Multifunctional Cu(II)–Dy(III) Enantiomers with Zero–Field Single–Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects. Molecules. 2023; 28(22):7506. https://doi.org/10.3390/molecules28227506
Chicago/Turabian StyleZhu, Shui-Dong, Yu-Lin Zhou, Fang Liu, Yu Lei, Sui-Jun Liu, He-Rui Wen, Bin Shi, Shi-Yong Zhang, Cai-Ming Liu, and Ying-Bing Lu. 2023. "A Pair of Multifunctional Cu(II)–Dy(III) Enantiomers with Zero–Field Single–Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects" Molecules 28, no. 22: 7506. https://doi.org/10.3390/molecules28227506
APA StyleZhu, S. -D., Zhou, Y. -L., Liu, F., Lei, Y., Liu, S. -J., Wen, H. -R., Shi, B., Zhang, S. -Y., Liu, C. -M., & Lu, Y. -B. (2023). A Pair of Multifunctional Cu(II)–Dy(III) Enantiomers with Zero–Field Single–Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects. Molecules, 28(22), 7506. https://doi.org/10.3390/molecules28227506