Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical-Chemical Profile of Liposomes
2.2. Antibacterial Activity and Efflux Pump Inhibition Assessed through MIC Reduction
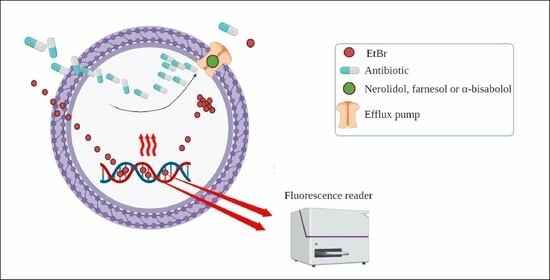
2.3. Evaluation of Efflux Pump Inhibition by Fluorescence Emission
3. Materials and Methods
3.1. Substances Used in Research
3.2. Synthesis of Liposomal Nanoformulations
3.3. Microorganisms Used in the Assays
3.4. Antibacterial Activity Evaluated by Measuring the Minimum Inhibitory Concentration (MIC)
3.5. Evaluation of the Inhibition of Efflux Pumps NorA, Tet(K), and MsrA
3.6. Efflux Pumps Inhibition Evaluated by the Increased Fluorescence Emission of EtBr
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Bello, C. Antibiotic Adjuvants—A Strategy to Unlock Bacterial Resistance to Antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Mangino, G.; Martellacci, L.; Quaranta, G.; Masucci, L.; Gallenzi, P. The Effect of Different Antibiotic Regimens on Bacterial Resistance: A Systematic Review. Antibiotics 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Pechère, J.C.; Plésiat, P. Bacterial Antibiotic Efflux Systems of Medical Importance. Cell. Mol. Life Sci. 1999, 56, 771–778. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ahn, J. Associations between Resistance Phenotype and Gene Expression in Response to Serial Exposure to Oxacillin and Ciprofloxacin in Staphylococcus aureus. Lett. Appl. Microbiol. 2017, 65, 462–468. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Kaatz, G.W.; McAleese, F.; Seo, S.M. Multidrug Resistance in Staphylococcus aureus Due to Overexpression of a Novel Multidrug and Toxin Extrusion (MATE) Transport Protein. Antimicrob. Agents Chemother. 2005, 49, 1857–1864. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Henderson, P.J.F.; Maher, C.; Elbourne, L.D.H.; Eijkelkamp, B.A.; Paulsen, I.T.; Hassan, K.A. Physiological Functions of Bacterial “Multidrug” Efflux Pumps. Chem. Rev. 2021, 121, 5417–5478. [Google Scholar] [CrossRef] [PubMed]
- Domingues Almeida, G.; Pena Godoi, E.; Cardoso Santos, E.; Paes De Lima, L.R.; Emilia De Oliveira, M. Extrato Aquoso de Allium sativum Potencializa a Ação Dos Antibióticos Vancomicina, Gentamicina e Tetraciclina Frente Staphylococcus aureus. Rev. Ciênc. Farm. Básica Apl. 2013, 34, 487–492. [Google Scholar]
- de Moura, D.F.; Rocha, T.A.; de Melo Barros, D.; da Silva, M.M.; dos Santos Santana, M.; Neta, B.M.; Cavalcanti, I.M.F.; Martins, R.D.; da Silva, M.V. Evaluation of the Antioxidant, Antibacterial, and Antibiofilm Activity of the Sesquiterpene Nerolidol. Arch. Microbiol. 2021, 203, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Tintino, C.D.d.M.; Santana, J.E.G.; Alencar, G.G.; Siqueira, G.M.; Gonçalves, S.A.; Tintino, S.R.; de Menezes, I.R.A.; Rodrigues, J.P.V.; Gonçalves, V.B.P.; Nicolete, R.; et al. Valencene, Nootkatone and Their Liposomal Nanoformulations as Potential Inhibitors of NorA, Tet(K), MsrA, and MepA Efflux Pumps in Staphylococcus aureus Strains. Pharmaceutics 2023, 15, 2400. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin. Transl. Med. 2017, 6, e44. [Google Scholar] [CrossRef]
- Liu, G.; Hou, S.; Tong, P.; Li, J. Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry. Crit. Rev. Anal. Chem. 2020, 52, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.; Auezova, L.; Danjou, P.E.; Fourmentin, S.; Greige-Gerges, H. First Evaluation of Drug-in-Cyclodextrin-in-Liposomes as an Encapsulating System for Nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef]
- Camilo, C.J.; Leite, D.O.D.; E Silva, A.R.A.; Menezes, I.R.A.; Coutinho, H.D.M.; da COSTA, J.G.M. Lipid Vesicles: Applications, Principal Components and Methods Used in Their Formulations: A Review. Acta Biol. Colomb. 2020, 25, 339–352. [Google Scholar] [CrossRef]
- Choudhury, A.; Sonowal, K.; Laskar, R.E.; Deka, D.; Dey, B.K. Liposome: A Carrier for Effective Drug Delivery. J. Appl. Pharm. Res. 2020, 8, 22–28. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent Advancements in Liposome Technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The Role of Liposomes in Clinical Nanomedicine Development. What Now? Now What? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of Flavonoids in Liposomal Delivery Systems: The Case of Quercetin, Kaempferol and Luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Rackova, L.; Firakova, S.; Kostalova, D.; Stefek, M.; Sturdik, E.; Majekova, M. Oxidation of Liposomal Membrane Suppressed by Flavonoids: Quantitative Structure-Activity Relationship. Bioorg. Med. Chem. 2005, 13, 6477–6484. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-Inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical Characteristics and In Vivo Deposition of Liposome-Encapsulated Tea Catechins by Topical and Intratumor Administrations. J. Drug Target. 2005, 13, 19–27. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as Multifunctional Nano-Carriers for Medicinal Natural Products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, D.W.; Lu, W.G.; Xu, H.; Yan, C.Y.; Hu, H.Y.; Chen, B.Y.; Qiao, M.X.; Zhao, X.L. Preparation, Characterization, and Evaluation of Liposomal Ferulic Acid In Vitro and In Vivo. Drug Dev. Ind. Pharm. 2008, 34, 602–608. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Mahmood, S.; Chatterjee, B.; Mandal, U.K. Pharmacokinetic Evaluation of the Synergistic Effect of Raloxifene Loaded Transfersomes for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102545. [Google Scholar] [CrossRef]
- Singh, S.; Verma, D.; Mirza, M.A.; Das, A.K.; Dudeja, M.; Anwer, M.K.; Sultana, Y.; Talegaonkar, S.; Iqbal, Z. Development and Optimization of Ketoconazole Loaded Nano-Transfersomal Gel for Vaginal Delivery Using Box-Behnken Design: In Vitro, Ex Vivo Characterization and Antimicrobial Evaluation. J. Drug Deliv. Sci. Technol. 2017, 39, 95–103. [Google Scholar] [CrossRef]
- Caster, J.M.; Yu, S.K.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.B.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of Particle Size on the Biodistribution, Toxicity, and Efficacy of Drug-Loaded Polymeric Nanoparticles in Chemoradiotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Kelidari, H.R.; Saeedi, M.; Morteza-Semnani, K.; Nabili, M.; Gohar, A.A.; Akbari, J.; Lotfali, E.; Nokhodchi, A. Time to Overcome Fluconazole Resistant Candida Isolates: Solid Lipid Nanoparticles as a Novel Antifungal Drug Delivery System. Colloids Surf. B Biointerfaces 2016, 142, 400–407. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Li, S.; Weng, Y.; Wang, K.; Zhang, T.; Chen, S.; Lu, X.; Jiang, Y.; Liang, J. Development and Validation of a Method for Determination of Encapsulation Efficiency of CPT-11/DSPE-MPEG2000 Nanoparticles. Med. Chem. 2016, 6, 345–348. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Hufschmid, R.; Teeman, E.; Mehdi, B.L.; Krishnan, K.M.; Browning, N.D. Observing the Colloidal Stability of Iron Oxide Nanoparticles In Situ. Nanoscale 2019, 11, 13098–13107. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Hoseini, B.; Jaafari, M.R.; Golabpour, A.; Momtazi-Borojeni, A.A.; Karimi, M.; Eslami, S. Application of Ensemble Machine Learning Approach to Assess the Factors Affecting Size and Polydispersity Index of Liposomal Nanoparticles. Sci. Rep. 2023, 13, 18012. [Google Scholar] [CrossRef]
- Minelli, R.; Serpe, L.; Pettazzoni, P.; Minero, V.; Barrera, G.; Gigliotti, C.L.; Mesturini, R.; Rosa, A.C.; Gasco, P.; Vivenza, N.; et al. Cholesteryl Butyrate Solid Lipid Nanoparticles Inhibit the Adhesion and Migration of Colon Cancer Cells. Br. J. Pharmacol. 2012, 166, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J. Active Methods of Drug Loading into Liposomes: Recent Strategies for Stable Drug Entrapment and Increased In Vivo Activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable Liposome-Encapsulated Hydrogels for Biomedical Applications: A Marriage of Convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and Thioxanthenes Inhibit Multidrug Efflux Pump Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of Drug Efflux Pumps in Staphylococcus aureus: Current Status of Potentiating Existing Antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef]
- DeMarco, C.E.; Cushing, L.A.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.A.; Kaatz, G.W. Efflux-Related Resistance to Norfloxacin, Dyes, and Biocides in Bloodstream Isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Falcão, C.; Viveiros, M.; MacHado, D.; Martins, M.; Melo-Cristino, J.; Amaral, L.; Couto, I. Exploring the Contribution of Efflux on the Resistance to Fluoroquinolones in Clinical Isolates of Staphylococcus aureus. BMC Microbiol. 2011, 11, 241. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Thomsen, V.F.; Martins, A.; Viveiros, M.; Amaral, L. Non-Antibiotics Reverse Resistance of Bacteria to Antibiotics. Vivo 2010, 24, 751–754. [Google Scholar]
- Patel, D.; Kosmidis, C.; Seo, S.M.; Kaatz, G.W. Ethidium Bromide MIC Screening for Enhanced Efflux Pump Gene Expression or Efflux Activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5070–5073. [Google Scholar] [CrossRef]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, R.M.A.; Sandle, T.; John, J.; Abuo-Rahma, G.E.D.A.; Hetta, H.F. A Novel Mechanism of Action of Ketoconazole: Inhibition of the Nora Efflux Pump System and Biofilm Formation in Multidrug-Resistant Staphylococcus aureus. Infect. Drug Resist. 2019, 12, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; Spengler, G.; Martins, A.; Martins, M.; Viveiros, M.; Mulhovo, S.; Ferreira, M.J.U.; Amaral, L. Inhibition of Efflux Pumps in Meticillin-Resistant Staphylococcus aureus and Enterococcus faecalis Resistant Strains by Triterpenoids from Momordica balsamina. Int. J. Antimicrob. Agents 2011, 37, 70–74. [Google Scholar] [CrossRef]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and Antioxidant Properties of Phytochemicals. Funct. Preserv. Prop. Phytochem. 2020, 1, 1–45. [Google Scholar] [CrossRef]
- Al Alsheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Kon, K.V.; Rai, M.K. Plant Essential Oils and Their Constituents in Coping with Multidrug-Resistant Bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Saavedra, M.J.; Borges, F.; Simões, M. Screening of Natural Molecules as Adjuvants to Topical Antibiotics to Treat Staphylococcus aureus from Diabetic Foot Ulcer Infections. Antibiotics 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, R.P.; de Freitas, T.S.; Costa, M.D.S.; Dos Santos, A.T.L.; Campina, F.F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; Araújo, A.A.D.S.; De Siqueira Júnior, J.P.; et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiotics 2020, 9, 28. [Google Scholar] [CrossRef]
- Barros, N.B.; Migliaccio, V.; Facundo, V.A.; Ciancaglini, P.; Stábeli, R.G.; Nicolete, R.; Silva-Jardim, I. Liposomal-Lupane System as Alternative Chemotherapy against Cutaneous Leishmaniasis: Macrophage as Target Cell. Exp. Parasitol. 2013, 135, 337–343. [Google Scholar] [CrossRef]
- Dutta, D.; Paul, B.; Mukherjee, B.; Mondal, L.; Sen, S.; Chowdhury, C.; Debnath, M.C. Nanoencapsulated Betulinic Acid Analogue Distinctively Improves Colorectal Carcinoma In Vitro and In Vivo. Sci. Rep. 2019, 9, 11506. [Google Scholar] [CrossRef]
- Alavi, S.E.; Koohi Moftakhari Esfahani, M.; Raza, A.; Adelnia, H.; Ebrahimi Shahmabadi, H. PEG-Grafted Liposomes for Enhanced Antibacterial and Antibiotic Activities: An In Vivo Study. NanoImpact 2022, 25, 100384. [Google Scholar] [CrossRef]
- Webb, M.S.; Boman, N.L.; Wiseman, D.J.; Saxon, D.; Sutton, K.; Wong, K.F.; Logan, P.; Hope, M.J. Antibacterial Efficacy against an In Vivo Salmonella typhimurium Infection Model and Pharmacokinetics of a Liposomal Ciprofloxacin Formulation. Antimicrob. Agents Chemother. 1998, 42, 45–52. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Zhang, W.; Zhao, X.; Li, H.; Xu, F.; Yin, L.; Peng, X.; Fu, H.; Chang, L.J.; et al. Preparation of Shikonin Liposome and Evaluation of Its In Vitro Antibacterial and In Vivo Infected Wound Healing Activity. Phytomedicine 2022, 99, 154035. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Piddock, L.J.V. How to Measure Export via Bacterial Multidrug Resistance Efflux Pumps. mBio 2016, 7, e00840-16. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A Novel Inhibitor of Multidrug Efflux Pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Markham, P.N.; Westhaus, E.; Klyachko, K.; Johnson, M.E.; Neyfakh, A.A. Multiple Novel Inhibitors of the NorA Multidrug Transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Tintino, C.D.d.M.; Tintino, S.R.; Muniz, D.F.; Rodrigues dos Santos Barbosa, C.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; et al. Chemical Synthesis, Molecular Docking and MepA Efflux Pump Inhibitory Effect by 1,8-Naphthyridines Sulfonamides. Eur. J. Pharm. Sci. 2021, 160, 105753. [Google Scholar] [CrossRef]
- CLSI CLSI, M100ED29E; Performance Standards for Antimicrobial Susceptibility Testing: 29th Informational Supplement 20. 29th ed. ANSI: Washington, DC, USA, 2019; ISBN 9781684400324.






| Liposome Control | Liposome/ Nerolidol | Liposome/ Farnesol | Liposome/ α-Bisabolol | |
|---|---|---|---|---|
| Size | 218.9 nm ± 45.1 | 241.8 nm ± 73.1 | 201.4 nm ± 66.6 | 183.5 nm ± 58 |
| Intensity | 2.5 a.u. | 3 a.u. | 5 a.u. | 5 a.u. |
| Zeta potential | −18.8 mV | −24.1 mV | −13 mV | 28.2 mV |
| pH | 7.4 | 7.4 | 7.4 | 7.4 |
| Concentration | 6.99 × 108 particles/mL | 6.82 × 108 particles/mL | 4.42 × 108 particles/mL | 4.02 × 108 particles/mL |
| Polydispersity index (PDI) | 0.50 | 0.61 | 0.92 | 0.755 |
| Encapsulation efficiency | - | 85.4% | 79% | 87% |
| Compound | 1199B | IS-58 | RN4220 |
|---|---|---|---|
| Nerolidol | 1024 µg/mL ± 0.5 | 32 µg/mL ± 0.5 * | 128 µg/mL ± 0.5 * |
| Farnesol | 25.4 µg/mL ± 0.8 * | 32 µg/mL ± 0.5 * | 16 µg/mL ± 0.5 * |
| α-bisabolol | 128 µg/mL ± 0.5 * | 64 µg/mL ± 0.5 * | 161.3 µg/mL ± 0.8 * |
| Liposome/Nerolidol | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 |
| Liposome/Farnesol | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 |
| Liposome/α-bisabolol | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 | ≥1024 µg/mL ± 0.5 |
| Group with Antibiotic | MIC | Group with EtBr | MIC |
|---|---|---|---|
| Norfloxacin | 50.8 µg/mL ± 0.8 | EtBr | 64 µg/mL ± 0.5 |
| Norfloxacin + CCCP | 32 µg/mL ± 0.5 * | EtBr + CCCP | 32 µg/mL ± 0.5 * |
| Norfloxacin + Nerolidol | 12.7 µg/mL ± 0.8 * | EtBr + Nerolidol | 5 µg/mL ± 0.5 * |
| Norfloxacin + Farnesol | 64 µg/mL ± 0.5 | EtBr + Farnesol | 50.8 µg/mL ± 0.8 * |
| Norfloxacin + α-bisabolol | 64 µg/mL ± 0.5 | EtBr + α-bisabolol | 80.6 µg/mL ± 0.8 |
| Norfloxacin + Liposome control | 101.6 µg/mL ± 0.8 | EtBr + Liposome control | 64 µg/mL ± 0.5 |
| Norfloxacin + Liposome/Nerolidol | 64 µg/mL ± 0.5 | EtBr + Liposome/Nerolidol | 64 µg/mL ± 0.5 |
| Norfloxacin + Liposome/Farnesol | 101.6 µg/mL ± 0.8 | EtBr + Liposome/Farnesol | 40.3 µg/mL ± 0.8 * |
| Norfloxacin + Liposome/α-bisabolol | 64 µg/mL ± 0.5 | EtBr + Liposome/α-bisabolol | 50.8 µg/mL ± 0.8 * |
| Group with Antibiotic | MIC | Group with EtBr | MIC |
|---|---|---|---|
| Tetracycline | 64 µg/mL ± 0.5 | EtBr | 10 µg/mL ± 0.5 |
| Tetracycline + CCCP | 25.4 µg/mL ± 0.8 * | EtBr + CCCP | 2 µg/mL ± 0.5 * |
| Tetracycline + Nerolidol | 64 µg/mL ± 0.5 | EtBr + Nerolidol | 25.4 µg/mL ± 0.8 |
| Tetracycline + Farnesol | 64 µg/mL ± 0.5 | EtBr + Farnesol | 10 µg/mL ± 0.5 |
| Tetracycline + α-bisabolol | 64 µg/mL ± 0.5 | EtBr + α-bisabolol | 8 µg/mL ± 0.5 * |
| Tetracycline + Liposome control | 128 µg/mL ± 0.5 | EtBr + Liposome control | 36 µg/mL ± 0.5 |
| Tetracycline + Liposome/Nerolidol | 256 µg/mL ± 0.5 | EtBr + Liposome/Nerolidol | 36 µg/mL ± 0.5 |
| Tetracycline + Liposome/Farnesol | 128 µg/mL ± 0.5 | EtBr + Liposome/Farnesol | 36 µg/mL ± 0.5 |
| Tetracycline + Liposome/α-bisabolol | 64 µg/mL ± 0.5 | EtBr + Liposome/α-bisabolol | 12.7 µg/mL ± 0.8 |
| Group with Antibiotic | MIC | Group with EtBr | MIC |
|---|---|---|---|
| Erythromycin | 512 µg/mL ± 0.5 | EtBr | 36 µg/mL ± 0.5 |
| Erythromycin + CCCP | 0.5 µg/mL ± 0.5 * | EtBr + CCCP | 2 µg/mL ± 0.5 * |
| Erythromycin + Nerolidol | 512 µg/mL ± 0.5 | EtBr + Nerolidol | 36 µg/mL ± 0.5 |
| Erythromycin + Farnesol | 256 µg/mL ± 0.5 * | EtBr + Farnesol | 21.8 µg/mL ± 0.8 * |
| Erythromycin + α-bisabolol | 512 µg/mL ± 0.5 | EtBr + α-bisabolol | 36 µg/mL ± 0.5 |
| Erythromycin + Liposome control | 512 µg/mL ± 0.5 | EtBr + Liposome control | 36 µg/mL ± 0.5 |
| Erythromycin + Liposome/Nerolidol | 512 µg/mL ± 0.5 | EtBr + Liposome/Nerolidol | 8 µg/mL ± 0.5 * |
| Erythromycin + Liposome/Farnesol | 512 µg/mL ± 0.5 | EtBr + Liposome/Farnesol | 21.8 µg/mL ± 0.8 * |
| Erythromycin + Liposome/α-bisabolol | 512 µg/mL ± 0.5 | EtBr + Liposome/α-bisabolol | 36 µg/mL ± 0.5 |
| Strain | Plasmid/Gene | Protein (Substrate Antibiotic) |
|---|---|---|
| 1199B | norA | NorA (Norfloxacin) |
| IS-58 | Plasmid PT181 (tetK) | Tet(K) (Tetracyclin) |
| RN4220 | Plasmid Pul5054 (msrA) | MsrA (Erythromycin) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, J.E.G.; Oliveira-Tintino, C.D.d.M.; Gonçalves Alencar, G.; Siqueira, G.M.; Sampaio Alves, D.; Moura, T.F.; Tintino, S.R.; de Menezes, I.R.A.; Rodrigues, J.P.V.; Gonçalves, V.B.P.; et al. Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations. Molecules 2023, 28, 7649. https://doi.org/10.3390/molecules28227649
Santana JEG, Oliveira-Tintino CDdM, Gonçalves Alencar G, Siqueira GM, Sampaio Alves D, Moura TF, Tintino SR, de Menezes IRA, Rodrigues JPV, Gonçalves VBP, et al. Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations. Molecules. 2023; 28(22):7649. https://doi.org/10.3390/molecules28227649
Chicago/Turabian StyleSantana, Jorge Ederson Gonçalves, Cícera Datiane de Morais Oliveira-Tintino, Gabriel Gonçalves Alencar, Gustavo Miguel Siqueira, Daniel Sampaio Alves, Talysson Felismino Moura, Saulo Relison Tintino, Irwin Rose Alencar de Menezes, João Pedro Viana Rodrigues, Vanessa Barbosa Pinheiro Gonçalves, and et al. 2023. "Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations" Molecules 28, no. 22: 7649. https://doi.org/10.3390/molecules28227649
APA StyleSantana, J. E. G., Oliveira-Tintino, C. D. d. M., Gonçalves Alencar, G., Siqueira, G. M., Sampaio Alves, D., Moura, T. F., Tintino, S. R., de Menezes, I. R. A., Rodrigues, J. P. V., Gonçalves, V. B. P., Nicolete, R., Emran, T. B., Gonçalves Lima, C. M., Ahmad, S. F., Coutinho, H. D. M., & da Silva, T. G. (2023). Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations. Molecules, 28(22), 7649. https://doi.org/10.3390/molecules28227649