Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction Analysis
2.2. FTIR Analysis
2.3. Raman Analysis
2.4. UV-Vis Diffuse Reflectance Spectra
2.5. Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst
2.6. Property Activity
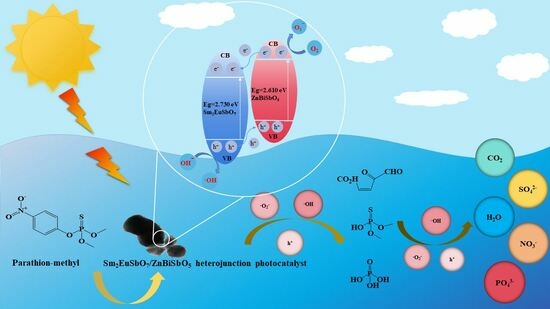
2.7. Analysis of Possible Degradation Mechanisms
3. Experimental Section
3.1. Materials and Reagents
3.2. Fabrication Method of ZnBiSbO5
3.3. Fabrication Method of Sm2EuSbO7
3.4. Fabrication of N-Doped TiO2
3.5. Fabrication of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst
3.6. Characterization
3.7. Photoelectrochemical Experiments
3.8. Experimental Setup and Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Yue, W.; Teng, Y.; Zhai, Y.; Zhu, H. Degradation kinetics and transformation pathway of methyl parathion by delta-MnO2/oxalic acid reaction system. Chemosphere 2023, 320, 138054. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.K.; Kaur, S.; Pal, B.; Singh, S. Morphological influence of ZnO nanostructures and their Cu loaded composites for effective photodegradation of methyl parathion. Solid State Sci. 2020, 99, 106045. [Google Scholar] [CrossRef]
- Zheng, L.; Pi, F.; Wang, Y.; Xu, H.; Zhang, Y.; Sun, X. Photocatalytic degradation of Acephate, Omethoate, and Methyl parathion by Fe3O4@SiO2@mTiO2 nanomicrospheres. J. Hazard. Mater. 2016, 315, 11–22. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Palestino, G.; de Lasa, H. Photocatalytic degradation of methyl parathion: Reaction pathways and intermediate reaction products. J. Photochem. Photobiol. A Chem. 2007, 186, 71–84. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Li, M.; Wang, M.; Liu, G.; Ping, J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. Trends Anal. Chem. 2023, 165, 117152. [Google Scholar] [CrossRef]
- An, X.; Chen, Y.; Ao, M.; Jin, Y.; Zhan, L.; Yu, B.; Wu, Z.; Jiang, P. Sequential photocatalytic degradation of organophosphorus pesticides and recovery of orthophosphate by biochar/α-Fe2O3/MgO composite: A new enhanced strategy for reducing the impacts of organophosphorus from wastewater. Chem. Eng. J. 2022, 435, 135087. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. In-depth biochemical identification of a novel methyl parathion hydrolase from Azohydromonas australica and its high effectiveness in the degradation of various organophosphorus pesticides. Bioresour. Technol. 2021, 323, 124641. [Google Scholar] [CrossRef]
- Liu, T.; Xu, S.; Lu, S.; Qin, P.; Bi, B.; Ding, H.; Liu, Y.; Guo, X.; Liu, X. A review on removal of organophosphorus pesticides in constructed wetland: Performance, mechanism and influencing factors. Sci. Total Environ. 2019, 651 Pt 2, 2247–2268. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Zawahry, M.; Emam, H.E. Efficient removal of organophosphorus pesticides from wastewater using polyethylenimine-modified fabrics. Polymer 2018, 155, 225–234. [Google Scholar] [CrossRef]
- Burrows, H.D.; Canle, M.; Santaballa, J.A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B-Biol. 2002, 67, 71–108. [Google Scholar] [CrossRef]
- Matus, P.; Littera, P.; Farkas, B.; Urik, M. Review on Performance of Aspergillus and Penicillium Species in Biodegradation of Organochlorine and Organophosphorus Pesticides. Microorganisms 2023, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Bouteh, E.; Ahmadi, N.; Abbasi, M.; Torabian, A.; van Loosdrecht, M.C.M.; Ducoste, J. Biodegradation of organophosphorus pesticides in moving bed biofilm reactors: Analysis of microbial community and biodegradation pathways. J. Hazard. Mater. 2021, 408, 124950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, Q.Y.; Rong, X.M.; Cai, P.; Liang, W.; Dai, K. Biodegradation of methyl parathion in the presence of goethite: The effect of Pseudomonas sp. Z1 adhesion. Int. Biodeterior. Biodegrad. 2014, 86, 294–299. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, W.; Liu, F.; Yue, X.; Liu, Y.; Yang, M.; Li, Z.; Wang, J. Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide. Chem. Eng. J. 2017, 313, 19–26. [Google Scholar] [CrossRef]
- Gatsios, E.; Hahladakis, J.N.; Gidarakos, E. Optimization of electrocoagulation (EC) process for the purification of a real industrial wastewater from toxic metals. J. Environ. Manag. 2015, 154, 117–127. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.; Yu, F.; Qiao, X.; Xu, Z. Simultaneous determination of ten organophosphate pesticide residues in fruits by gas chromatography coupled with magnetic separation. J. Sep. Sci. 2014, 37, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, S.; Jin, L.; Zhang, C.; Liu, W. Enantiomeric separation of organophosphorus pesticides by high-performance liquid chromatography, gas chromatography and capillary electrophoresis and their applications to environmental fate and toxicity assays. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.M.; Han, H.T.; Wang, Y.X.; Liu, S.T.; Zhao, J.Y.; Meng, X.C.; Li, Z.Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Dong, S.Y.; Cui, L.F.; Zhang, W.; Xia, L.J.; Zhou, S.J.; Russell, C.K.; Fan, M.H.; Feng, J.L.; Sun, J.H. Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Zhang, F.B.; Wang, X.M.; Liu, H.N.; Liu, C.L.; Wan, Y.; Long, Y.Z.; Cai, Z.Y. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Wu, J.; Ren, J.X.; Pan, W.G.; Lu, P.; Qi, Y.F. The Photocatalytic Technology for Wastewater Treatment. In Photo-Catalytic Control Technologies of Flue Gas Pollutants; Springer: Singapore, 2019; pp. 141–150. [Google Scholar]
- Raja, V.; Shiamala, L.; Alamelu, K.; Ali, B.M.J. A study on the free radical generation and photocatalytic yield in extended surfaces of visible light active TiO2 compounds. Sol. Energy Mater. Sol. Cells 2016, 152, 125–132. [Google Scholar] [CrossRef]
- Li, X.Y.; Hou, Y.; Zhao, Q.D.; Wang, L.Z. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Chandrasekaran, A. Recent advances in TiO2/ZnS-based binary and ternary photocatalysts for the degradation of organic pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2 and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Yang, T.; Peng, J.; Zheng, Y.; He, X.; Hou, Y.; Wu, L.; Fu, X. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl. Catal. B 2018, 221, 223–234. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Xing, J.; Xing, Y.; Meng, A. One step synthesis of Co/Cr-codoped ZnO nanoparticle with superb adsorption properties for various anionic organic pollutants and its regeneration. J. Hazard. Mater. 2018, 352, 204–214. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.H.; Sha, J.W.; Zhong, C.; Hua, W.B.; Zhao, N.Q. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Zhang, L.H.; Fan, M.H.; Wang, X.X.; Walbridge, M.L.; Nong, Q.Y.; Wu, Y.; Zhao, L.H. Z-scheme SnO2−x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, X.F.; Luan, D.Y.; Lou, X.W. Fabrication of Heterostructured Fe2TiO5-TiO2 Nanocages with Enhanced Photoelectrochemical Performance for Solar Energy Conversion. Angew. Chem. Int. Ed. 2020, 59, 8128–8132. [Google Scholar] [CrossRef]
- Li, J.H.; Han, M.S.; Guo, Y.; Wang, F.; Sun, C. Fabrication of FeVO4/Fe2TiO5 composite catalyst and photocatalytic removal of norfloxacin. Chem. Eng. J. 2016, 298, 300–308. [Google Scholar] [CrossRef]
- Luan, J.F.; Li, Y.Y. Photocatalytic Water Splitting for Hydrogen Production with Gd2MSbO7 (M = Fe, In, Y) Photocatalysts under Visible Light Irradiation. Materials 2015, 8, 16–30. [Google Scholar] [CrossRef]
- Wan, C.L.; Qu, Z.X.; Du, A.B.; Pan, W. Influence of B site substituent Ti on the structure and thermophysical properties of A2B2O7-type pyrochlore Gd2Zr2O7. Acta Mater. 2009, 57, 4782–4789. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Arakawa, H. Growth, photophysical and structural properties of Bi2InNbO7. J. Cryst. Growth 2001, 229, 462–466. [Google Scholar] [CrossRef]
- Bahareh, K.; Habibi, M.H. High photocatalytic activity of light-driven Fe2TiO5 nanoheterostructure toward degradation of antibiotic metronidazole. J. Ind. Eng. Chem. 2019, 80, 292–300. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, H.; Zhao, Y.; Li, G.; Li, Z. Preparation, Characterization of A2Ce2O7 (A = La and Gd) and Their Photo-Catalytic Properties. Energy Environ. Focus 2015, 4, 324–329. [Google Scholar]
- Wang, J.H.; Zou, Z.G.; Ye, J.H. Synthesis, structure and photocatalytic property of a new hydrogen evolving photocatalyst Bi2InTaO7. Mater. Sci. Forum. 2003, 423, 485–490. [Google Scholar] [CrossRef]
- Selvaraj, S.; Patrick, D.S.; Vangari, G.A.; Mohan, M.K.; Ponnusamy, S.; Muthamizchelvan, C. Facile synthesis of Sm doped ZnO nanoflowers by Co-precipitation method for enhanced photocatalytic degradation of MB dye under sunlight irradiation. Ceram. Int. 2022, 48, 29049–29058. [Google Scholar]
- Zong, Y.Q.; Li, Z.; Wang, X.M.; Ma, J.T.; Men, Y. Synthesis and high photocatalytic activity of Eu-doped ZnO nanoparticles. Ceram. Int. 2014, 40, 10375–10382. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.; Elhouichet, H.; Férid, M. Preparation, characterization of Sb-doped ZnO nanocrystals and their excellent solar light driven photocatalytic activity. Appl. Surf. Sci. 2017, 393, 486–495. [Google Scholar] [CrossRef]
- Zhu, B.C.; Cheng, B.; Fan, J.J.; Ho, W.K.; Yu, J.G. g-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Shen, T.; Shi, X.K.; Guo, J.X.; Li, J.; Yuan, S.D. Photocatalytic removal of NO by light-driven Mn3O4/BiOCl heterojunction photocatalyst: Optimization and mechanism. Chem. Eng. J. 2021, 408, 128014. [Google Scholar] [CrossRef]
- Jiang, T.G.; Wang, K.; Guo, T.; Wu, X.Y.; Zhang, G.K. Fabrication of Z-scheme MoO3/Bi2O4 heterojunction photocatalyst with enhanced photocatalytic performance under visible light irradiation. Chin. J. Catal. 2020, 41, 161–169. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.J.; Yuan, J.L.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J.M. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Zuo, G.; Ma, S.; Yin, Z.; Chen, W.; Wang, Y.; Ji, Q.; Xian, Q.; Yang, S.; He, H. Z-Scheme Modulated Charge Transfer on InVO4@ZnIn2S4 for Durable Overall Water Splitting. Small 2023, 19, 2207031. [Google Scholar] [CrossRef] [PubMed]
- Sohrabian, M.; Mahdikhah, V.; Alimohammadi, E.; Sheibani, S. Improved photocatalytic performance of SrTiO3 through a Z-scheme polymeric-perovskite heterojunction with g-C3N4 and plasmonic resonance of Ag mediator. Appl. Surf. Sci. 2023, 618, 156682. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, C.Y.; Liu, H.; Li, P.; Wei, F.F.; Jiang, Y.; Song, W.G. A Bi/BiOCl heterojunction photocatalyst with enhanced electron–hole separation and excellent visible light photodegrading activity. J. Mater. Chem. A 2014, 2, 1677–1681. [Google Scholar] [CrossRef]
- Wang, W.J.; Yu, J.C.; Xia, D.H.; Wong, P.K.; Li, Y.C. Graphene and g-C3N4 Nanosheets Cowrapped Elemental α-Sulfur as a Novel Metal-Free Heterojunction Photocatalyst for Bacterial Inactivation under Visible-Light. Environ. Sci. Technol. 2013, 47, 8724–8732. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A novel Z-scheme Ag3VO4/BiVO4 heterojunction photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Catal. B 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Lu, H.; Hao, Q.; Chen, T.; Zhang, L.; Chen, D.; Ma, C.; Yao, W.; Zhu, Y. A high-performance Bi2O3/Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B 2018, 237, 59–67. [Google Scholar] [CrossRef]
- Zangiabadi, M.; Mehrabi, F.; Nasiripur, P.; Baghersad, M.H. Visible-light-driven photocatalytic degradation of methyl parathion as chemical warfare agent simulant by NiO/Bi2MoO6 heterojunction photocatalyst. J. Mol. Struct. 2022, 1256, 132472. [Google Scholar] [CrossRef]
- Ramacharyulu, P.; Kumar, J.P.; Prasad, G.K.; Srivastava, A.R. Synthesis, characterization and photocatalytic activity of Ag-TiO2 nanoparticulate film. Rsc Adv. 2015, 5, 1309–1314. [Google Scholar] [CrossRef]
- Ikram, M.; Hassan, J.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Qamar, U.; Ali, S. Photocatalytic and bactericidal properties and molecular docking analysis of TiO2 nanoparticles conjugated with Zr for environmental remediation. Rsc Adv. 2020, 10, 30007–30024. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Abe, R.; Arakawa, H. Photocatalytic decomposition of water with Bi2InNbO7. Catal. Lett. 2000, 68, 235–239. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water Splitting into H2 and O2 on New Sr2M2O7 (M = Nb and Ta) Photocatalysts with Layered Perovskite Structures: Factors Affecting the Photocatalytic Activity. J. Phys. Chem. B 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Kohno, M.; Ogura, S.; Sato, K.; Inoue, Y. Properties of photocatalysts with tunnel structures: Formation of a surface lattice O- radical by the UV irradiation of BaTi4O9 with a pentagonal-prism tunnel structure. Chem. Phys. Lett. 1997, 267, 72–76. [Google Scholar] [CrossRef]
- Mohan, S.; Kaur, S.; Singh, D.P.; Kaur, P. Structural and luminescence properties of samarium doped lead alumino borate glasses. Opt. Mater. 2017, 73, 223–233. [Google Scholar] [CrossRef]
- Goh, K.H.; Haseeb, A.S.M.A.; Wong, Y.H. Effect of Oxidation Temperature on Physical and Electrical Properties of Sm2O3 Thin-Film Gate Oxide on Si Substrate. J. Electron. Mater. 2016, 45, 5302–5312. [Google Scholar] [CrossRef]
- Şabikoğlu, İ.; Paralı, L. FTIR and VSM properties of samarium-doped nickel ferrite. Funct. Mater. 2014, 7, 1450046. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Leontie, L.; Doroftei, C.; Carlescu, A.; Bulai, G.; Mothudi, B.; Zorkani, I.; Jorio, A. Microstructural, FTIR and Raman spectroscopic study of Rare earth doped ZnO nanostructures. Mater. Today Proc. 2022, 53, 319–323. [Google Scholar] [CrossRef]
- Bosca, M.; Pop, L.; Borodi, G.; Pascuta, P.; Culea, E. XRD and FTIR structural investigations of erbium-doped bismuth–lead–silver glasses and glass ceramics. J. Alloys Compd. 2009, 479, 579–582. [Google Scholar] [CrossRef]
- Pascuta, P.; Culea, E. FTIR spectroscopic study of some bismuth germanate glasses containing gadolinium ions. Mater. Lett. 2008, 62, 4127–4129. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Sajan, D.; Devarajan, P.A. A rapid and versatile method for solvothermal synthesis of Sb2O3 nanocrystals under mild conditions. Appl. Nanosci. 2012, 3, 529–533. [Google Scholar] [CrossRef]
- Rada, S.; Rus, L.; Rada, M.; Zagrai, M.; Culea, E.; Rusu, T. Compositional dependence of structure, optical and electrochemical properties of antimony(III) oxide doped lead glasses and vitroceramics. Ceram. Int. 2014, 40, 15711–15716. [Google Scholar] [CrossRef]
- Ji, L.; Chen, N.; Du, G.; Yan, M.; Shi, W. Synthesis and luminescence of Y2O3:Eu3+ inorganic–organic hybrid nanostructures with thenoyltrifluoroacetone. Ceram. Int. 2014, 40, 3117–3122. [Google Scholar] [CrossRef]
- Patel, D.K.; Vishwanadh, B.; Sudarsan, V.; Kulshreshtha, S.K.; McKittrick, J. Difference in the Nature of Eu3+ Environment in Eu3+-Doped BaTiO3 and BaSnO3. J. Am. Ceram. Soc. 2013, 96, 3857–3861. [Google Scholar] [CrossRef]
- Janani, B.; Okla, M.K.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Thomas, A.M.; Raju, L.L.; Al-Qahtani, W.H.; Khan, S.S. CuO loaded ZnS nanoflower entrapped on PVA-chitosan matrix for boosted visible light photocatalysis for tetracycline degradation and anti-bacterial application. J. Environ. Manag. 2022, 306, 114396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Sasmal, K.; Sun, Y.Y.; Chu, C.W. Raman spectroscopy of RFeAsO(R=Sm, La). Phys. Rev. B 2008, 77, 220505. [Google Scholar] [CrossRef]
- Tsaryuk, V.I.; Zhuravlev, K.P.; Szostak, R.; Vologzhanina, A.V. Structure, Luminescence, and Raman Spectroscopy of Europium and Terbium Dipivaloylmethanates and Other β-Diketonates with 2,2′-Bipyridine. J. Struct. Chem. 2020, 61, 1026–1037. [Google Scholar] [CrossRef]
- Refat, M.S.; Elsabawy, K.M. Infrared spectra, Raman laser, XRD, DSC/TGA and SEM investigations on the preparations of selenium metal, (Sb2O3, Ga2O3, SnO and HgO) oxides and lead carbonate with pure grade using acetamide precursors. Bull. Mater. Sci. 2011, 34, 873–881. [Google Scholar] [CrossRef]
- Gilliam, S.J.; Jensen, J.O.; Banerjee, A.; Zeroka, D.; Kirkby, S.J.; Merrow, C.N. A theoretical and experimental study of Sb4O6: Vibrational analysis, infrared, and Raman spectra. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2004, 60, 425–434. [Google Scholar] [CrossRef]
- Sukriti; Chand, P.; Singh, V. Enhanced visible-light photocatalytic activity of samarium-doped zinc oxide nanostructures. J. Rare Earths 2020, 38, 29–38. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Zhang, Q.W.; Qu, J.; Ai, Z.Q.; Li, Y.J. Mechanochemical synthesis of ultrafine ZnS/Zn-Al layered double hydroxide heterojunction and their photocatalytic activities in dye degradation. Appl. Clay Sci. 2017, 144, 115–120. [Google Scholar] [CrossRef]
- Chahine, A.; Et-tabirou, M.; Pascal, J.L. FTIR and Raman spectra of the Na2O–CuO–Bi2O3–P2O5 glasses. Mater. Lett. 2004, 58, 2776–2780. [Google Scholar] [CrossRef]
- Domoratskii, K.V.; Pastukhov, V.I.; Kudzin, A.Y.; Sadovskaya, L.Y.; Rizak, V.M.; Stefanovich, V.A. Raman scattering in the Bi2TeO5 single crystal. Phys. Solid State 2000, 42, 1443–1446. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S.; Eibschutz, M. Photoelectrolysis with YFeO3 electrodes. J. Appl. Phys. 1977, 48, 3070–3072. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Gohar, R.S.; Ehsan, M.F.; Karamat, N.; Najam-Ul-Haq, M.; Shah, A.; Nisar, J.; Qureshi, A.M.; Ashiq, M.N. Photomineralization of untreated wastewater by a novel LaCeZr2O7-SnSe nanocomposite as a visible light driven heterogeneous photocatalyst. Solid State Sci. 2020, 106, 106305. [Google Scholar] [CrossRef]
- Deng, B.; Tran, V.; Xie, Y.; Jiang, H.; Li, C.; Guo, Q.; Wang, X.; Tian, H.; Koester, S.J.; Wang, H.; et al. Efficient electrical control of thin-film black phosphorus bandgap. Nat. Commun. 2017, 8, 14474. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zeng, P.; Liu, P.; Zhao, C.; Xie, W.; Mai, W. Interface Engineering To Boost Photoresponse Performance of Self-Powered, Broad-Bandwidth PEDOT:PSS/Si Heterojunction Photodetector. ACS Appl. Mater. Interfaces 2016, 8, 19158–19167. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Bao, J. Interfacial charge transfer of heterojunction photocatalysts: Characterization and calculation. Surf. Interfaces 2021, 25, 101265. [Google Scholar] [CrossRef]
- Luan, J.F.; Niu, B.W.; Ma, B.B.; Yang, G.M.; Liu, W.L. Preparation and Property Characterization of In2YSbO7/BiSnSbO6 Heterojunction Photocatalyst toward Photocatalytic Degradation of Indigo Carmine within Dye Wastewater under Visible-Light Irradiation. Materials 2022, 15, 6648. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.F.; Ma, B.B.; Yao, Y.; Liu, W.L.; Niu, B.W.; Yang, G.M.; Wei, Z.J. Synthesis, Performance Measurement of Bi2SmSbO7/ZnBiYO4 Heterojunction Photocatalyst and Photocatalytic Degradation of Direct Orange within Dye Wastewater under Visible Light Irradiation. Materials 2022, 15, 3986. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hu, J.; He, J.; Huang, X.; Hu, N.; Li, Y.; Huang, Q.; Guo, S.; Liu, X.; Yang, Z.; et al. Direct Z-scheme hierarchical heterostructures of oxygen-doped g-C3N4/In2S3 with efficient photocatalytic Cr(vi) reduction activity. Catal. Sci. Technol. 2021, 11, 7963–7972. [Google Scholar] [CrossRef]
- Liu, B.Y.; Du, J.Y.; Ke, G.L.; Jia, B.; Huang, Y.J.; He, H.C.; Zhou, Y.; Zou, Z.G. Boosting O2 Reduction and H2O Dehydrogenation Kinetics: Surface N-Hydroxymethylation ofg-C3N4 Photocatalysts for the Efficient Production of H2O2. Adv. Funct. Mater. 2022, 32, 2111125. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Y.; Han, Z.T.; Sun, Y.; Wang, X.Q.; Zhang, Q.F.; Zou, Z.G. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2021, 42, 164–174. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, M.S.; Haider, A.; Shahzadi, I.; Mustajab, M.; Ul-Hamid, A.; Shahzadi, A.; Nabgan, W.; Algaradah, M.M.; Fouda, A.M.; et al. Co-precipitated vanadium oxide-doped carbon spheres and graphene oxide nanorods serve as antimicrobial and catalytic agents: In silico molecular docking study. J. Alloys Compd. 2023, 961, 171045. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Ren, W.W.; Yang, J.K.; Zhang, J.X.; Li, W.; Sun, C.Y.; Zhao, H.L.; Wen, Y.T.; Sha, O.; Liang, B. Recent progress in SnO2/g-C3N4 heterojunction photocatalysts: Synthesis, modification, and application. J. Alloys Compd. 2022, 906, 164372. [Google Scholar] [CrossRef]
- Yaacob, N.; Sean, G.P.; Nazri, N.A.M.; Ismail, A.F.; Abidin, M.N.Z.; Subramaniam, M.N. Simultaneous oily wastewater adsorption and photodegradation by ZrO2-TiO2 heterojunction photocatalysts. J. Water Process Eng. 2021, 39, 101644. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhou, M.H.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.J.; Wang, W.J.; Chen, J.H.; Liu, R.R.; Xie, S.; Wang, J.; Ji, Z.J. Synthesis of octahedral-like ZnO/ZnFe2O4 heterojunction photocatalysts with superior photocatalytic activity. Solid State Sci. 2019, 96, 105901. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.N.; Sarrouf, S.; Ehsan, M.F.; Manzoor, S.; Ashiq, M.N.; Alshawabkeh, A.N. Polarity reversal for enhanced in-situ electrochemical synthesis of H2O2 over banana-peel derived biochar cathode for water remediation. Electrochim. Acta 2023, 453, 142351. [Google Scholar] [CrossRef]
- Prasad, G.K.; Ramacharyulu, P.V.R.K.; Kumar, J.P.; Srivastava, A.R.; Singh, B. Photocatalytic degradation of paraoxon-ethyl in aqueous solution using titania nanoparticulate film. Thin Solid Film. 2012, 520, 5597–5601. [Google Scholar] [CrossRef]
- Aghaei, M.; Sajjadi, S.; Keihan, A.H. Sono-coprecipitation synthesis of ZnO/CuO nanophotocatalyst for removal of parathion from wastewater. Environ. Sci. Pollut. Res. Int. 2020, 27, 11541–11553. [Google Scholar] [CrossRef]
- Nasiripur, P.; Zangiabadi, M.; Baghersad, M.H. Visible light photocatalytic degradation of methyl parathion as chemical warfare agents simulant via GO-Fe3O4/Bi2MoO6 nanocomposite. J. Mol. Struct. 2021, 1243, 130875. [Google Scholar] [CrossRef]
- Mardiroosi, A.; Mahjoub, A.R.; Fakhri, H.; Boukherroub, R. Design and fabrication of a perylene dimiide functionalized g-C3N4@UiO-66 supramolecular photocatalyst: Insight into enhancing the photocatalytic performance. J. Mol. Struct. 2021, 1246, 131244. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Mujasam Batoo, K.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Behera, A.; Mansingh, S.; Das, K.K.; Parida, K. Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr (VI) reduction. J. Colloid Interface Sci. 2019, 544, 96–111. [Google Scholar] [CrossRef]
- Mardiroosi, A.; Mahjoub, A.R.; Khavar, A.H.C.; Boukherroub, R.; Sillanpaeae, M.; Kaur, P. Effects of functionalized magnetic graphene oxide on the visible-light-induced photocatalytic activity of perovskite-type MTiO3 (M = Zn and Mn) for the degradation of Rhodamine B. J. Mol. Struct. 2023, 1284, 135298. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Xu, S.; Gong, S.; Jiang, H.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. Z-scheme heterojunction through interface engineering for broad spectrum photocatalytic water splitting. Appl. Catal. B 2020, 267, 118661. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Arakawa, H. Structural properties of InNbO4 and InTaO4: Correlation with photocatalytic and photophysical properties. Chem. Phys. Lett. 2000, 332, 271–277. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.F.; Fu, Q.M.; Chen, M. Fabrication of a novel biochar decorated nano-flower-like MoS2 nanomaterial for the enhanced photodegradation activity of ciprofloxacin: Performance and mechanism. Mater. Res. Bull. 2022, 147, 111650. [Google Scholar] [CrossRef]
- Yan, S.W.; Yang, J.; Li, Y.; Jia, X.H.; Song, H.J. One-step synthesis of ZnS/BiOBr photocatalyst to enhance photodegradation of tetracycline under full spectral irradiation. Mater. Lett. 2020, 276, 128232. [Google Scholar] [CrossRef]
- Zhang, J.F.; Hu, Y.F.; Jiang, X.L.; Chen, S.F.; Meng, S.G.; Fu, X.L. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]


























| Atom | x | y | z | Occupied Index |
|---|---|---|---|---|
| Sm | 0 | 0 | 0 | 1 |
| Eu | 0.5 | 0.5 | 0.5 | 0.5 |
| Sb | 0.5 | 0.5 | 0.5 | 0.5 |
| O(1) | −0.175 | 0.125 | 0.125 | 1 |
| O(2) | 0.125 | 0.125 | 0.125 | 1 |
| Atom | x | y | z | Occupied Index |
|---|---|---|---|---|
| Zn | 0.5 | 0.5 | 0.5 | 1 |
| Bi | 0 | 0 | 0 | 1 |
| Sb | 0.5 | 0.5 | 0.5 | 1 |
| O(1) | 0.125 | 0.125 | 0.125 | 0.5 |
| O(2) | 0.125 | 0.125 | 0.375 | 1 |
| Photocatalyst | Radiation | Irradiation Time (min) | Pesticide | Removal Rate (%) | Ref. |
|---|---|---|---|---|---|
| TiO2 | UV | 300 | Parathion methyl | 90 | [108] |
| ZnO/CuO | Simulated solar light | 100 | Parathion methyl | 90 | [109] |
| ZnO nanorod | Visible light | 180 | Parathion methyl | 99 | [2] |
| Bi2MoO6 | Visible light | 120 | Parathion methyl | 69 | [58] |
| NiO/Bi2MoO6 | Visible light | 120 | Parathion methyl | 95 | [58] |
| GO-Fe2O3 | Visible light | 140 | Parathion methyl | 40 | [110] |
| GO-Fe2O3/Bi2MoO6 | Visible light | 140 | Parathion methyl | 98 | [110] |
| Ag-TiO2 | Visible light | 420 | Parathion methyl | 100 | [59] |
| Sm2EuSbO7 | Visible light | 150 | Parathion methyl | 92 | This study |
| SZHP | Visible light | 150 | Parathion methyl | 100 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Hao, L.; Yao, Y.; Wang, Y.; Yang, G.; Li, J. Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation. Molecules 2023, 28, 7722. https://doi.org/10.3390/molecules28237722
Luan J, Hao L, Yao Y, Wang Y, Yang G, Li J. Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation. Molecules. 2023; 28(23):7722. https://doi.org/10.3390/molecules28237722
Chicago/Turabian StyleLuan, Jingfei, Liang Hao, Ye Yao, Yichun Wang, Guangmin Yang, and Jun Li. 2023. "Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation" Molecules 28, no. 23: 7722. https://doi.org/10.3390/molecules28237722
APA StyleLuan, J., Hao, L., Yao, Y., Wang, Y., Yang, G., & Li, J. (2023). Preparation and Property Characterization of Sm2EuSbO7/ZnBiSbO5 Heterojunction Photocatalyst for Photodegradation of Parathion Methyl under Visible Light Irradiation. Molecules, 28(23), 7722. https://doi.org/10.3390/molecules28237722