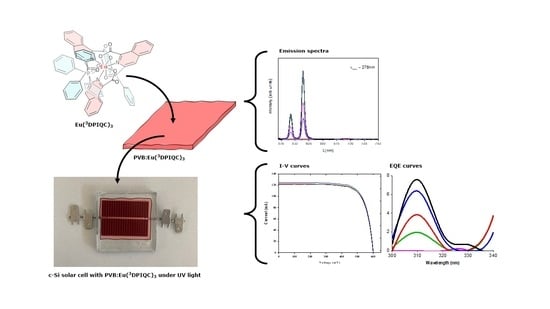
Enhancing c-Si Solar Cell Efficiency in the UV Region: Photophysical Insights into the Use of Eu3+ Complexes for Down-Shifting Layer Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Optical Properties of the Eu(3DPIQC)3 Complex in Solution and in PVB Films
2.3. Addition of an LDSL to a c-Si Solar Cell
2.4. Electrochemical Impedance Spectra
3. Materials and Methods
3.1. Characterization Techniques
3.2. Computational Modelling
3.3. Preparation of the Polymeric Luminescent Films
3.4. Device Fabrication and Characterization
- Wet chemical alkaline saw damage etching: The wafers underwent a wet chemical alkaline etching process to remove the damage caused by sawing. Subsequently, they were thoroughly cleaned.
- P-doping profiles for the n+ type back surface field (BSF): The sample was prepared using POCl3 diffusion in a quartz tube furnace. This profile, denoted as P-BSF, achieves a surface concentration of 6 × 1019 cm−3 with a junction depth of approximately 0.45 μm.
- Silicon nitride (SiNx) deposition: After the POCl3 diffusion process for the BSF, a silicon nitride layer was deposited on the rear side using plasma-enhanced chemical vapor deposition (PECVD).
- Wet chemical alkaline texturing: The wafer was subjected to a wet chemical alkaline texturing process to enhance light absorption. This step was followed by another cleaning process.
- Formation of the p-n junction: On the front side, the p-n junction was formed through BBr3 diffusion, which created the necessary doping for the formation of the junction.
- Passivation/antireflection coatings: The passivation and antireflection coatings were applied to the front side of the solar cells. These coatings consisted of stacks made of thermal silicon dioxide and silicon nitride (SiO2/SiNx). The thermal silicon dioxide layer was achieved through thermal oxidation, while the silicon nitride layer was deposited using PECVD.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Bouwman, E. Synthesis and Photophysical Properties of a Highly Luminescent EuIII-Containing Hybrid Thin Film. Polyhedron 2016, 118, 25–29. [Google Scholar] [CrossRef]
- Tanner, P.A. Some Misconceptions Concerning the Electronic Spectra of Tri-Positive Europium and Cerium. Chem. Soc. Rev. 2013, 42, 5090–5101. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Kumar, S.; Sen, S.; Sundararajan, K. Photoluminescence Studies of Ln3+ (Ln = Eu, Tb) Doped Y(Benzoate)3 Complexes. J. Lumin. 2023, 262, 119950. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, J.; Lei, S.; Hu, W. Long Afterglow MOFs: A Frontier Study on Synthesis and Applications. Mater. Chem. Front. 2021, 5, 6824–6849. [Google Scholar] [CrossRef]
- Mehare, M.D.; Mehare, C.M.; Swart, H.C.; Dhoble, S.J. Recent Development in Color Tunable Phosphors: A Review. Prog. Mater. Sci. 2023, 133, 101067. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Jaiswal, A.; Suman, S.; Jaiswal, R.P. Luminescent Down-Shifting Natural Dyes to Enhance Photovoltaic Efficiency of Multicrystalline Silicon Solar Module. Sol. Energy 2020, 206, 353–364. [Google Scholar] [CrossRef]
- Correia, S.F.H.; Bastos, A.R.N.; Fu, L.S.; Carlos, L.D.; André, P.S.; Ferreira, R.A.S. Lanthanide-Based Downshifting Layers Tested in a Solar Car Race. Opto-Electron. Adv. 2019, 2, 190006. [Google Scholar] [CrossRef]
- Voiculescu, A.M.; Hau, S.; Stanciu, G.; Avram, D.; Gheorghe, C. Optical Thermometry through Infrared Excited Green Upconversion Emissions of Er3+-Yb3+ Co-Doped LaAlO3 Phosphors. J. Lumin. 2022, 242, 118602. [Google Scholar] [CrossRef]
- Du, P.; Guo, Y.; Lee, S.H.; Yu, J.S. Broad Near-Ultraviolet and Blue Excitation Band Induced Dazzling Red Emissions in Eu3+-Activated Gd2MoO6 Phosphors for White Light-Emitting Diodes. RSC Adv. 2017, 7, 3170–3178. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Tian, X.; Wang, L.; Wu, W.; Luo, Y.; Zhang, Q. Europium Complex Doped Luminescent Solar Concentrators with Extended Absorption Range from UV to Visible Region. Sol. Energy 2011, 85, 2179–2184. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing Solar Cell Efficiency: The Search for Luminescent Materials as Spectral Converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Ma, W.; Luo, Y.; Wang, L.; Hu, Z.; Wu, W.; Wang, X.; Zou, G.; Zhang, Q. Luminescent Solar Concentrator Employing Rare Earth Complex with Zero Self-Absorption Loss. Sol. Energy 2011, 85, 2571–2579. [Google Scholar] [CrossRef]
- Hosseini, Z.; Ghanbari, T. Designing an Efficient Graphene Quantum Dot-Filled Luminescent down Shifting Layer to Improve the Stability and Efficiency of Perovskite Solar Cells by Simple Optical Modeling. RSC Adv. 2018, 8, 31502–31509. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Lemus, R.; Sanchiz, J.; Sierra-Ramos, M.; Martín, I.R.; Hernández-Rodríguez, C.; Borchert, D. Downshifting Maximization Procedure Applied to [Eu(Bphen)(Tta)3] at Different Concentrations Applied to a Photovoltaic Device and Covered with a Hemispherical Reflector. Sens. Actuators A Phys. 2018, 271, 60–65. [Google Scholar] [CrossRef]
- Müller, B.; Hardt, L.; Armbruster, A.; Kiefer, K.; Reise, C. Yield Predictions for Photovoltaic Power Plants: Empirical Validation, Recent Advances and Remaining Uncertainties. Prog. Photovolt. Res. Appl. 2016, 24, 570–583. [Google Scholar] [CrossRef]
- Brito-Santos, G.; Hernández-Rodríguez, C.; Gil-Hernández, B.; Sanchiz, J.; Martín, I.R.; González-Díaz, B.; Guerrero-Lemus, R. Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells. Materials 2023, 16, 5068. [Google Scholar] [CrossRef] [PubMed]
- Satpute, N.S.; Mehare, C.M.; Tiwari, A.; Swart, H.C.; Dhoble, S.J. Synthesis and Luminescence Characterization of Downconversion and Downshifting Phosphor for Efficiency Enhancement of Solar Cells: Perspectives and Challenges. ACS Appl. Electron. Mater. 2022, 4, 3354–3391. [Google Scholar] [CrossRef]
- Wang, Y.; Gawryszewska-Wilczynsk, P.; Zhang, X.; Yin, J.; Wen, Y.; Li, H. Photovoltaic Efficiency Enhancement of Polycrystalline Silicon Solar Cells by a Highly Stable Luminescent Film. Sci. China Mater. 2020, 63, 544–551. [Google Scholar] [CrossRef]
- Yang, D.; Liang, H.; Liu, Y.; Hou, M.; Kan, L.; Yang, Y.; Zang, Z. A Large-Area Luminescent Downshifting Layer Containing an Eu3+ Complex for Crystalline Silicon Solar Cells. Dalt. Trans. 2020, 49, 4725–4731. [Google Scholar] [CrossRef]
- Maggioni, G.; Campagnaro, A.; Tonezzer, M.; Carturan, S.; Quaranta, A. Deposition and Characterization of Luminescent Eu(Tta)3phen-Doped Parylene-Based Thin-Film Materials. ChemPhysChem 2013, 14, 1853–1863. [Google Scholar] [CrossRef]
- Kai, J.; Felinto, M.C.F.C.; Nunes, L.A.O.; Malta, O.L.; Brito, H.F. Intermolecular Energy Transfer and Photostability of Luminescence-Tuneable Multicolour PMMA Films Doped with Lanthanide-β-Diketonate Complexes. J. Mater. Chem. 2011, 21, 3796–3802. [Google Scholar] [CrossRef]
- Moudam, O.; Rowan, B.C.; Alamiry, M.; Richardson, P.; Richards, B.S.; Jones, A.C.; Robertson, N. Europium Complexes with High Total Photoluminescence Quantum Yields in Solution and in PMMA. Chem. Commun. 2009, 6649–6651. [Google Scholar] [CrossRef]
- Dong, J.; Lin, B. Photoelectric Efficiency Enhancement of a Polycrystalline Silicon Solar Cell Coated with an EVA Film Containing Eu3+ Complex by Addition of Modified SiO2. RSC Adv. 2016, 6, 110409–110415. [Google Scholar] [CrossRef]
- Ruby, D.S.; Kent, S.W. The Effects of Concentrated Ultraviolet Light of High-Efficiency Silicon Solar Cells. In Proceedings of the The Conference Record of the Twenty-Second IEEE Photovoltaic Specialists Conference, Las Vegas, NV, USA, 7–11 October 1991; Volume 1, pp. 111–117. [Google Scholar] [CrossRef]
- Sinha, A.; Qian, J.; Moffitt, S.L.; Hurst, K.; Terwilliger, K.; Miller, D.C.; Schelhas, L.T.; Hacke, P. UV-Induced Degradation of High-Efficiency Silicon PV Modules with Different Cell Architectures. Prog. Photovolt. Res. Appl. 2023, 31, 36–51. [Google Scholar] [CrossRef]
- Park, S.H.; Ahn, S.; Gwak, J.; Shin, K.; Ahn, S.K.; Yoon, K.; Cho, Y.; Kim, D.W.; Yun, J.H. Effectiveness of Full Spectrum Light Soaking on Solar Cell Degradation Analysis. Curr. Appl. Phys. 2013, 13, 1684–1688. [Google Scholar] [CrossRef]
- Katsagounos, G.; Stathatos, E.; Arabatzis, N.B.; Keramidas, A.D.; Lianos, P. Enhanced Photon Harvesting in Silicon Multicrystalline Solar Cells by New Lanthanide Complexes as Light Concentrators. J. Lumin. 2011, 131, 1776–1781. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, C.; Sun, B.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Luminescent Europium(III) Complexes Based on Tridentate Isoquinoline Ligands with Extremely High Quantum Yield. Inorg. Chem. Front. 2021, 8, 41–47. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 2013206534. [Google Scholar] [CrossRef]
- Regalado-Pérez, E.; Mathews, N.R.; Mathew, X. Eu(III) Complex-Polymer Composite Luminescence down-Shifting Layers for Reducing the Blue-Losses in Thin Film Solar Cells. Sol. Energy 2020, 199, 82–91. [Google Scholar] [CrossRef]
- Malta, O.L.; Brito, H.F.; Menezes, J.F.S.; Gonçalves E Silva, F.R.; Alves, S.; Farias, F.S.; De Andrade, A.V.M. Spectroscopic Properties of a New Light-Converting Device Eu(Thenoyltrifluoroacetonate)3 2(Dibenzyl Sulfoxide). A Theoretical Analysis Based on Structural Data Obtained from a Sparkle Model. J. Lumin. 1997, 75, 255–268. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectroscopy. Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yang, R.D.; Li, F.S.; Yu, K.B. Crystal Structure and Antitumor Activity of Some Rare Earth Metal Complexes with Schiff Base. Polyhedron 2000, 19, 2599–2604. [Google Scholar] [CrossRef]
- Tao, C.; Yuan, X.; Yin, Q.; Yan, H.; Ni, W.; Yan, L.; Zhang, L. Synthesis, Characterization and Photoluminescent Properties of Europium(III) Complexes with Ligands Bearing Benzimidazole Groups. J. Mater. Sci. Mater. Electron. 2016, 27, 5715–5722. [Google Scholar] [CrossRef]
- Dutra, J.D.L.; Bispo, T.D.; Freire, R.O. LUMPAC Lanthanide Luminescence Software: Efficient and User Friendly. J. Comput. Chem. 2014, 35, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Filho, M.A.M.; Dutra, J.D.L.; Rocha, G.B.; Freire, R.O.; Simas, A.M. Sparkle/RM1 Parameters for the Semiempirical Quantum Chemical Calculation of Lanthanide Complexes. RSC Adv. 2013, 3, 16747–16755. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Atom Clusters and Supramolecular Architectures. Dalt. Trans. 2008, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Crosby, G.A.; Whan, R.E.; Alire, R.M. Intramolecular Energy Transfer in Rare Earth Chelates. Role of the Triplet State. J. Chem. Phys. 1961, 34, 743–748. [Google Scholar] [CrossRef]
- Zhou, Y.; Pang, M.; Zhu, W.; Jiang, Z.; Liu, Y.; Meng, J. Crystal Structure and Photochromism of Auxochrome-Introduced Spiro[Indoline-Quinoline]Oxazine Deriatives. J. Mol. Struct. 2020, 1219, 128574. [Google Scholar] [CrossRef]
- Fuentes, S.; Barraza, N.; Veloso, E.; Villarroel, R.; Llanos, J. Effects of Eu Substitution on Luminescent and Magnetic Properties of BaTiO3 Nanomaterials. J. Alloys Compd. 2013, 569, 52–57. [Google Scholar] [CrossRef]
- King, A.; Singh, R.; Nayak, B.B. Synthesis and Photoluminescence Behaviour of Ultra-Fine Particles of Eu-Doped Zirconia Nanopowders. J. Solid State Chem. 2020, 282, 121106. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, S.; Hu, S.; Zhang, L.; Liu, L. Electrospinning Preparation and Luminescence Properties of Eu(TTA)3phen/Polystyrene Composite Nanofibers. J. Rare Earths 2010, 28, 333–339. [Google Scholar] [CrossRef]
- Gorller-Walrand, C.; Binnemans, K. Spectral Intensities of f-f Transitions. Handb. Phys. Chem. Rare Earths 1998, 25, 101–264. [Google Scholar] [CrossRef]
- Araújo, A.A.S.; Brito, H.F.; Malta, O.L.; Matos, J.R.; Teotonio, E.E.S.; Storpirtis, S.; Izumi, C.M.S. Synthesis and Photophysical Study of Highly Luminescent Coordination Compounds of Rare Earth Ions with Thenoyltrifluoroacetonate and AZT. J. Inorg. Biochem. 2002, 88, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pothuganti, P.K.; Bhogi, A.; Kalimi, M.R.; Reniguntla, P. Physical and Optical Properties of Borobismuthate Glasses Containing Vanadium Oxide. Glas. Phys. Chem. 2020, 46, 146–154. [Google Scholar] [CrossRef]
- Judd, B.R. Interaction with William Carnall. J. Solid State Chem. 2005, 178, 408–411. [Google Scholar] [CrossRef]
- Fujita, S.; Umayahara, Y.; Tanabe, S. Influence of Light Scattering on Luminous Efficacy in Ce: YAG Glass-Ceramic Phosphor. J. Ceram. Soc. Jpn. 2010, 118, 128–131. [Google Scholar] [CrossRef]
- Ho, W.J.; Liu, J.J.; Ke, B.X. Characterization of Luminescent Down-Shifting Spectral Conversion Effects on Silicon Solar Cells with Various Combinations of Eu-Doped Phosphors. Materials 2022, 15, 452. [Google Scholar] [CrossRef]
- Ho, W.J.; You, B.J.; Liu, J.J.; Bai, W.B.; Syu, H.J.; Lin, C.F. Photovoltaic Performance Enhancement of Silicon Solar Cells Based on Combined Ratios of Three Species of Europium-Doped Phosphors. Materials 2018, 11, 845. [Google Scholar] [CrossRef]
- Fix, T.; Nonat, A.; Imbert, D.; Di Pietro, S.; Mazzanti, M.; Charbonnière, L.J.; Slaoui, A. Enhancement of Silicon Solar Cells by Downshifting with Eu and Tb Coordination Complexes. Prog. Photovolt. Res. Appl. 2016, 24, 1251–1260. [Google Scholar] [CrossRef]
- Le Donne, A.; Dilda, M.; Crippa, M.; Acciarri, M.; Binetti, S. Rare Earth Organic Complexes as Down-Shifters to Improve Si-Based Solar Cell Efficiency. Opt. Mater. 2011, 33, 1012–1014. [Google Scholar] [CrossRef]
- Le Donne, A.; Acciarri, M.; Narducci, D.; Marchionna, S.; Binetti, S. Encapsulating Eu3+ Complex Doped Layers to Improve Si-Based Solar Cell Efficiency. Prog. Photovolt. Res. Appl. 2009, 17, 519–525. [Google Scholar] [CrossRef]
- Markose, K.K.; Jasna, M.; Subha, P.P.; Antony, A.; Jayaraj, M.K. Performance Enhancement of Organic/Si Solar Cell Using CNT Embedded Hole Selective Layer. Sol. Energy 2020, 211, 158–166. [Google Scholar] [CrossRef]
- Srivastava, A.; Sharma, D.; Srivastava, S.K. Impedance Spectroscopy Analysis to Probe the Role of Interface Properties of Surface Micro-Engineered PEDOT:PSS/n-Si Solar Cells. Org. Electron. 2023, 119, 106817. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC2012; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2012. [Google Scholar]
- Ridley, J.E.; Zerner, M.C. Triplet States via Intermediate Neglect of Differential Overlap: Benzene, Pyridine and the Diazines. Theor. Chim. Acta 1976, 42, 223–236. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Opelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Dutra, J.D.L.; Lima, N.B.D.; Freire, R.O.; Simas, A.M. Europium Luminescence: Electronic Densities and Superdelocalizabilities for a Unique Adjustment of Theoretical Intensity Parameters. Sci. Rep. 2015, 5, srep13695. [Google Scholar] [CrossRef] [PubMed]
- Carneiro Neto, A.N.; Teotonio, E.E.S.; de Sá, G.F.; Brito, H.F.; Legendziewicz, J.; Carlos, L.D.; Felinto, M.C.F.C.; Gawryszewska, P.; Moura, R.T.; Longo, R.L.; et al. Modeling Intramolecular Energy Transfer in Lanthanide Chelates: A Critical Review and Recent Advances. Handb. Phys. Chem. Rare Earths 2019, 56, 55–162. [Google Scholar] [CrossRef]
- Alemany, P.; Casanova, D.; Alvarez, S.; Dryzun, C.; Avnir, D. Continuous Symmetry Measures: A New Tool in Quantum Chemistry. In Reviews in Computational Chemistry; Parrill, A.L., Lipkowitz, K.B., Eds.; WILEY: Hoboken, NJ, USA, 2017; Volume 30, pp. 289–352. [Google Scholar] [CrossRef]
- Alemany, P.; Bernuz, E.; Carreras, A.; Llunell, M. Cosymlib: A Python library for continuous symmetry measures (v0.9.5). Zenodo 2021. [Google Scholar] [CrossRef]
- Olivares, D.; Ferrada, P.; Marzo, A.; Pinto, K.; Espinoza, D.; Rabanal-Arabach, J.; Portillo, C.; Fuentealba, E.; Llanos, J. Study of the Effects of Soiling on PV Devices Using the Spin-Coating Technique in Accelerated Indoor Exposures. Sol. Energy 2022, 231, 317–327. [Google Scholar] [CrossRef]
- Zakirova, G.G.; Mladentsev, D.Y.; Borisova, N.E. Palladium-Catalyzed C-P Cross-Coupling between (Het)aryl Halides and Secondary Phosphine Oxides. Synthesis 2019, 51, 2379–2386. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wu, G.J.; Li, Y.; Gao, L.X.; Han, F.S. [NiCl2(dppp)]-catalyzed cross-coupling of aryl halides with dialkyl phosphite, diphenylphosphine oxide, and diphenylphosphine. Chem. A Eur. J. 2012, 18, 9622–9627. [Google Scholar] [CrossRef]










| Sample | Ω2 | Ω4 | Ω6 | ARAD | ANRAD |
|---|---|---|---|---|---|
| Eu(3DPIQC)3 (calc.) | 4.13 | 0.49 | 0.03 | 166.24 | 288.30 |
| Eu(3DPIQC)3 (exp.) | 4.14 | 0.48 | - | 181.51 | 273.04 |
| PVB: Eu(3DPIQC)3 (2%) | 4.39 | 2.00 | - | 211.52 | 243.05 |
| PVB: Eu(3DPIQC)3 (4%) | 4.49 | 1.38 | - | 205.24 | 249.30 |
| PVB: Eu(3DPIQC)3 (6%) | 4.60 | 0.96 | - | 202.32 | 255.22 |
| PVB: Eu(3DPIQC)3 (8%) | 4.71 | 0.90 | - | 204.95 | 249.60 |
| Eu Complex Concentration (%) | Jsc (mA/cm2) | Voc (mV) | FF (%) | (%) | (%) | EQE (%) 300–330 [nm] | EQE (%) 330–400 [nm] | EQE (%) 400–1000 [nm] | Jsc cal. (mA/cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 30.44 | 600.0 | 69% | 12.60 | -- | 0.02 | 34.27 | 86.57 | 35.28 |
| 2 | 30.56 | 600.7 | 69% | 12.67 | 0.6 | 0.67 | 34.48 | 86.77 | 35.43 |
| 4 | 30.50 | 600.2 | 69% | 12.63 | 0.2 | 1.11 | 34.17 | 86.43 | 35.30 |
| 6 | 31.10 | 600.9 | 69% | 12.89 | 2.3 | 2.26 | 32.23 | 85.76 | 40.17 |
| 8 | 30.43 | 599.9 | 69% | 12.60 | 0.0 | 2.69 | 30.11 | 85.27 | 39.89 |
| LSC Layer | EQE Increase | Ref. |
|---|---|---|
| Eu-doped phosphor/SiO2 | 8% | [50] |
| [Eu(tta)3(tppo)2]/EVA | 19% | [51] |
| [EuL3]/EVA | 15% | [51] |
| [Eu(tfc)3:EABP] 1:1 EVA | 5% | [52] |
| [Eu(tfc)3/Eu(dbm)3phen]/PVA | 5% | [53] |
| Eu(3DPIQC)3/PVB | 8% | This work |
| Sample | R1 (mΩ) | R2 (mΩ) | C1(F) × 10−8 |
|---|---|---|---|
| Bare Cell | 129 | 200 | 1.98 |
| PVB: Eu(3DPIQC)3 (6%) | 129 | 276 | 2.00 |
| PVB: Eu(3DPIQC)3 (8%) | 129 | 250 | 1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, F.; Nelson, R.; Espinoza, D.; Brito, I.; Sánchez-Muñoz, L.; Alemany, P.; Ortiz, S.; Ferrada, P.; Mestra, A.; Llanos, J. Enhancing c-Si Solar Cell Efficiency in the UV Region: Photophysical Insights into the Use of Eu3+ Complexes for Down-Shifting Layer Applications. Molecules 2023, 28, 7924. https://doi.org/10.3390/molecules28237924
Vargas F, Nelson R, Espinoza D, Brito I, Sánchez-Muñoz L, Alemany P, Ortiz S, Ferrada P, Mestra A, Llanos J. Enhancing c-Si Solar Cell Efficiency in the UV Region: Photophysical Insights into the Use of Eu3+ Complexes for Down-Shifting Layer Applications. Molecules. 2023; 28(23):7924. https://doi.org/10.3390/molecules28237924
Chicago/Turabian StyleVargas, Fabian, Ronald Nelson, Dario Espinoza, Ivan Brito, Laura Sánchez-Muñoz, Pere Alemany, Sergio Ortiz, Pablo Ferrada, Alifhers Mestra, and Jaime Llanos. 2023. "Enhancing c-Si Solar Cell Efficiency in the UV Region: Photophysical Insights into the Use of Eu3+ Complexes for Down-Shifting Layer Applications" Molecules 28, no. 23: 7924. https://doi.org/10.3390/molecules28237924
APA StyleVargas, F., Nelson, R., Espinoza, D., Brito, I., Sánchez-Muñoz, L., Alemany, P., Ortiz, S., Ferrada, P., Mestra, A., & Llanos, J. (2023). Enhancing c-Si Solar Cell Efficiency in the UV Region: Photophysical Insights into the Use of Eu3+ Complexes for Down-Shifting Layer Applications. Molecules, 28(23), 7924. https://doi.org/10.3390/molecules28237924