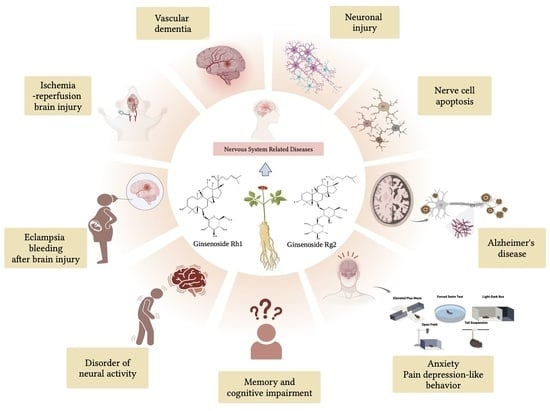
Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms
Abstract
:1. Introduction
2. Effect of Ginsenoside Rg2 on Nervous System
2.1. Protective Effect on Nerve Cells
2.2. Treatment of Vascular Dementia
2.3. Anti-Ischemia Reperfusion Brain Injury
2.4. Anti-Anxiety, Anti-Pain Depression-like Behavior
2.5. Anti-Alzheimer’s Disease
2.6. Protective Effect on Neuronal Injury
2.7. Improvement of Brain Injury after Bleeding in Eclampsia Models
3. Effect of Ginsenoside Rh1 on Nervous System
3.1. The Regulatory Effect on Neural Activity
3.2. Improve Memory and Cognitive Impairment
3.3. Anti-Alzheimer’s Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | parkinson’s disease |
| DA | dopamine |
| 6-OHDA | 6-Hydroxydopamine hydrobromide |
| ERK | extracellular signal-regulated protein kinase |
| JNK | c-jun N-terminal kinase |
| NB | neuroblastoma |
| MDA | malondialdehyde |
| NO | nitric oxide |
| Glu | glutamic acid |
| NMDA | N-Methyl-d-aspartic acid |
| APP | amyloid Precursor Protein |
| Aβ1-40 | amyloid β-Protein (1-40) |
| Aβ25-35 | amyloid β-Protein (25-35) |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X |
| AChE | Acetylcholinesterase |
| ChAT | Choline acetyltransferase |
| ACh | Acetylcholine |
| SY | synaptophysin |
| ICH | intracerebral hemorrhage |
| TLR4 | toll-like Receptor 4 |
| NF-κB | nuclear factor kappa-B |
| SD | Sleep deprivation |
| HPA | hypothalamic-pituitary-adrenal |
| HPG | hypothalamic-pituitary-gonadal |
| MyD88 | myeloiddifferentiationfactor88 |
| PI3K | Phosphatidylinositide 3-kinases |
| Akt | Protein kinase B |
| VD | Vascular dementia |
| CICR | cerebral ischemia-reperfusion injury |
| HSP70 | heat shock protein 70 |
| P53 | recombinant Tumor Protein |
| OGD/R | Oxygen glucose stripping/Reperfusion |
| CCI | chronic constric-tion injury |
| BDNF | brain-derived neurotrophic factor |
| TrkB | Tyrosine Kinase receptor B |
| shRNA | short hairpin RNA |
| PTSD | Post-traumatic stress disorder |
| SPS | single-long stress |
| EPMT | elevated plus maze test |
| 5-HT | 5-hydroxytryptamine |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| CRH | corticotropin releasing hormone |
| Cort | corticosterone |
| ACTH | adrenocorticotropic hormone |
| AD | Alzheimer’s disease |
| CNS | Central Nervous System |
| CN | Calcineurin |
| NFAT | Nuclear factor activating T cells |
| SP | Schizophrenia |
| CCI | Cardio-cerebral infarction |
| p-IκBα | phospho-IκBα |
References
- Im, D.-S.; Nah, S.-Y. Yin and Yang of ginseng pharmacology: Ginsenosides vs. gintonin. Acta Pharmacol. Sin. 2013, 34, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, P.; Yang, W.; Zhao, C.; Zhang, L.; Zhang, J.; Qin, Y.; Xu, H.; Huang, L. Characterization of the Components and Pharmacological Effects of Mountain-Cultivated Ginseng and Garden Ginseng Based on the Integrative Pharmacology Strategy. Front. Pharmacol. 2021, 12, 659954. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.; Wang, Y. Advances in Saponin Diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Zang, H. Study on the Preparation Method of Primary Panaxtriol Secondary Ginsenosides and Sapogenin. Master’s Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]
- Mi, J.; Zhang, M.; Ren, G.; Zhang, H.; Wang, Y.; Hu, P. Enriched separation of protopanaxatriol ginsenosides, malonyl ginsenosides and protopanaxadiol ginsenosides from Panax ginseng using macroporous resins. J. Food Eng. 2012, 113, 577–588. [Google Scholar] [CrossRef]
- Mou, N.; Duan, Z.; Ma, P.; Fu, R.; Fan, D. Study on the hypnotic effect of rare protopanaxadiol-type and protopanaxatriol-type ginsenosides. RSC Adv. 2019, 9, 20483–20491. [Google Scholar] [CrossRef]
- Mhgyzhzyyhlszlh, Z. Advances on effects of ginsenoside Rg2 and ginsenoside Rh1 on nervous system and related mechanisms. Shanghai J. Tradit. Chin. Med. 2018, 52, 110–112. [Google Scholar]
- Turale, S.; Thana, K. Global Challenges in Caregiving for Older Adults: Solutions and Call to Action. J. Gerontol. Nurs. 2023, 49, 3–6. [Google Scholar] [CrossRef]
- Kumar, N.; Saraber, P.; Ding, Z.; Kusumbe, A.P. Diversity of Vascular Niches in Bones and Joints During Homeostasis, Ageing, and Diseases. Front. Immunol. 2021, 12, 798211. [Google Scholar] [CrossRef]
- Bhusal, A.; Rahman, M.H.; Suk, K. Hypothalamic inflammation in metabolic disorders and aging. Cell. Mol. Life Sci. 2021, 79, 32. [Google Scholar] [CrossRef]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Yoon, Y.-S.; Lee, S.-J. Molecular Mechanisms of Cellular Senescence in Neurodegenerative Diseases. J. Mol. Biol. 2023, 435, 168114. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; Darweesh, S.K.L.; Wolters, F.J.; Fani, L.; Heshmatollah, A.; Mutlu, U.; Koudstaal, P.J.; Heeringa, J.; Leening, M.I.G.; Ikram, M.K.; et al. Lifetime risk of common neurological diseases in the elderly population. J. Neurol. Neurosurg. Psychiatry 2019, 90, 148. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, R.; Nam, S.M.; Kim, D.G.; Cho, I.H.; Kim, H.C.; Cho, Y.J.; Rhim, H.; Nah, S.Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr. Med. Res. 2021, 10, 100450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Gao, W.; Wu, X.; Zheng, M.; Yu, Y.; Song, C.; Miao, W.; Yang, Z.; He, Y.; Liu, C.; et al. Ginsenoside Rg2 Ameliorates High-Fat Diet-Induced Metabolic Disease through SIRT1. J. Agric. Food Chem. 2020, 68, 4215–4226. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Costa, K.M.; Schoenbaum, G. Dopamine. Curr. Biol. 2022, 32, R817–R824. [Google Scholar] [CrossRef]
- Cruces-Sande, A.; Rodríguez-Pérez, A.I.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Méndez-Álvarez, E.; Labandeira-García, J.L.; Soto-Otero, R. Copper Increases Brain Oxidative Stress and Enhances the Ability of 6-Hydroxydopamine to Cause Dopaminergic Degeneration in a Rat Model of Parkinson’s Disease. Mol. Neurobiol. 2019, 56, 2845–2854. [Google Scholar] [CrossRef]
- Beleslin, D.B.; Samardžić, R.; Krstić, S.K. 6-Hydroxydopamine-induced aggression in cats: Effects of various drugs. Pharmacol. Biochem. Behav. 1986, 24, 1821–1823. [Google Scholar] [CrossRef]
- Ratner, N.; Brodeur, G.M.; Dale, R.C.; Schor, N.F. The “neuro” of neuroblastoma: Neuroblastoma as a neurodevelopmental disorder. Ann. Neurol. 2016, 80, 13–23. [Google Scholar] [CrossRef]
- Li, X.-F.; Lui, C.N.-P.; Jiang, Z.-H.; Ken, Y.K.-L. Neuroprotective effects of ginsenosides Rh1 and Rg2 on neuronal cells. Chin. Med. 2011, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Sun, L.; Zhao, X.; Zhang, S.; Gong, S.; Wang, J.; Yu, H. Mechanism of Rg2 underlying Aβ1-40 aggregation in rats following ischemia/reperfusion injury. Chin. J. Geriatr. Heart Brain Vessel. Dis. 2014, 16, 78–80. [Google Scholar]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Ganguly, J.; Pal, S.; Ghosal, M. Pattern of cognitive deficits in vascular dementia. Indian J. Med. Res. 2019, 149, 503. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, A.; Zhou, Y.; San, X.; Jin, T.; Jin, Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J. Ethnopharmacol. 2008, 115, 441–448. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Z.Y.; Jin, Y.; Long, C.L.; Wang, H. Effect of ginsenoside-Rg2 on learning and memory of vascular dementia rats. Chin. J. Clin. Pharmacol. Ther. 2008, 3, 276–282. [Google Scholar]
- Tat, H. Ischemic Cerebrovascular Disease. Brain 2002, 125, 2782–2783. [Google Scholar]
- Chen, M.; Wu, Y.; Zhang, Y.; Jin, Y. Neuroprotective effects of ginsenoside Rg2 on cerebral ischemia reperfusion injury in rats. J. Apoplexy Nerv. Dis. 2014, 31, 1089–1092. [Google Scholar]
- Li, T.; Lu, G.; Cui, Y.; Zhang, G.; Jin, Y. Effects of ischemia reperfusion on expression of amyloid and amyloid precursor protein in rat hippocampal neu- rons and the intervention of ginsenoside Rg2. J. Apoplexy Nerv. Dis. 2008, 25, 417–420. [Google Scholar]
- Gao, Y.; Li, R.; Sun, H.; Li, J.; He, B.; Xiao, S.; Li, L.; Wang, J. Protective Effects of Oroxylin A on Oxygen-Glucose Deprivation/Reperfusion-Induced PC12 Cells by Activating the Sonic Hedgehog Signal Pathway. Nat. Prod. Commun. 2019, 14, 1934578X19881544. [Google Scholar] [CrossRef]
- Pi, M.S.; Ru, Q.; Gong, X.K.; Wu, R.H.; Tian, X.; Qi, X.; Li, C.Y. Effect of Ginsenoside Rg2 and Its Stereoisomers on Oxygen-Glucose Deprivation and Reperfusion Induced Cortical Neuronal Injury Model. Chin. J. Integr. Tradit. West. Med. 2016, 36, 333–338. [Google Scholar]
- Egorova, E.; Nikitina, N.; Rebrov, A. Ab0864-Hpr Influence of The Type of Pain Syndrome on the Severity of Anxiety-Depressive Disorders In Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80 (Suppl. S1), 1455–1456. [Google Scholar] [CrossRef]
- Berrahal, I.; Ayadi, B.; Haddad, M. Depression among Chronic Pain Patients. Eur. Psychiatry 2017, 41, S708. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Li, S.Y.; Li, P. Effects of ginsenoside-Rg2 on mechanical allodynia, heat hyperalgeia, depressive state of rats with chronic sciatic nerve constriction injury. Chin. J. Appl. Physiol. 2019, 35, 228–231. [Google Scholar]
- Fedorová, S.; Blažková, M.; Humpolíček, P.; Barteček, R. Cognitive Impairment in Major Depressive Disorder and Severe Depressive Episode with Psychotic Symptoms. Eur. Psychiatry 2017, 41, S143–S144. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Nardi, B.; Bonelli, C.; Gravina, D.; Benedetti, F.; Amatori, G.; Simone Battaglini, S.; Massimetti, G.; Luciano, M.; Berardelli, I.; et al. Investigating suicidality across the autistic-catatonic continuum in a clinical sample of subjects with major depressive disorder and borderline personality disorder. Front. Psychiatry 2023, 14, 1124241. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.-L.; Zhang, X.; Wang, H.; Ye, Y.; Song, L. Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res. Bull. 2017, 134, 211–219. [Google Scholar] [CrossRef]
- Longo, L.; Jannini, T.; Merlo, M.; Cecora, V.; Gagliano, M.; D’Imperia, B.; Daverio, A.; Monaco, L.; Rossi, I.; Niolu, C.; et al. Suicidality in post-traumatic stress disorder (PTSD) and complex PTSD (CPTSD). Eur. Psychiatry 2021, 64, S142. [Google Scholar] [CrossRef]
- Maercker, A.; Cloitre, M.; Bachem, R.; Schlumpf, Y.R.; Khoury, B.; Hitchcock, C.; Bohus, M. Complex post-traumatic stress disorder. Lancet 2022, 400, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sanchís-Ollé, M.; Belda, X.; Gagliano, H.; Visa, J.; Nadal, R.; Armario, A. Animal models of PTSD: Comparison of the neuroendocrine and behavioral sequelae of immobilization and a modified single prolonged stress procedure that includes immobilization. J. Psychiatr. Res. 2023, 160, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sur, B.; Lee, B. Ginsenoside Rg3 modulates spatial memory and fear memory extinction by the HPA axis and BDNF-TrkB pathway in a rat post-traumatic stress disorder. J. Nat. Med. 2022, 76, 821–831. [Google Scholar] [CrossRef]
- Gao, Z.-w.; Ju, R.-L.; Luo, M.; Wu, S.-l.; Zhang, W.-T. The anxiolytic-like effects of ginsenoside Rg2 on an animal model of PTSD. Psychiatry Res. 2019, 279, 130–137. [Google Scholar]
- Brookmeyer, R.; Abdalla, N.; Kawas, C.H.; Corrada, M.M. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dement. 2018, 14, 121–129. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lutz, M.W.; Xing, Y. A systems-based model of Alzheimer’s disease. Alzheimers Dement. 2019, 15, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Chen, Q.; Yan, P.; Liu, R.; Liang, H.; Zhang, M. Effect of Ginsenosides on Prevention of Alzheimer’s Disease. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 022004. [Google Scholar] [CrossRef]
- Zhuang, Y.; Shi, B.; Tian, X.; Cui, L. Effects of ginsenoside Rg2 on learning and memory ability and age spot formation in Alzheimer’s disease model rats. Chin. J. Gerontol. 2010, 30, 202–204. [Google Scholar]
- Ming-Liang, S.-Z. Influence of ginsenoside-Rg2 on scopolamine-induced learning and memory impairment in mice and its mechanism. J. Chin. Pract. Diagn. Ther. 2017, 31, 444–447. [Google Scholar]
- Wu, C.; Zhuang, Y.; Li, Y.; Cui, L. Effects of ginsenoside Rg2 on hippocampal neuron structure and synaptophysin expression in rats with Alzheimer’s disease. Chin. J. Gerontol. 2012, 5, 989–992. [Google Scholar]
- Shuangyan, W.; Ruowu, S.; Hongli, N.; Bei, Z.; Yong, S. Protective Effects of Rg2 on Hypoxia-induced Neuronal Damage in Hippocampal Neurons. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; Costa, F.D.S.C.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Park, Y.; Cho, G.J.; Kim, L.Y.; Lee, T.-S.; Oh, M.-J.; Kim, Y.-H. Preeclampsia Increases the Incidence of Postpartum Cerebrovascular Disease in Korean Population. J. Korean Med. Sci. 2018, 33, e35. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Factors linked to increased stroke risk are identified in women with pre-eclampsia. BMJ 2017, 357, j2606. [Google Scholar] [CrossRef]
- Cai, L.; Hu, F.; Fu, W.; Yu, X.; Zhong, W.; Liu, F.; Wang, T.; Sui, D. Ginsenoside Rg2 Ameliorates Brain Injury After Intracerebral Hemorrhage in a Rat Model of Preeclampsia. Reprod. Sci. 2021, 28, 3431–3439. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Lee, J.; Choi, M.-K.; Song, I.-S. Pharmacokinetics of ginsenosides following repeated oral administration of red ginseng extract significantly differ between species of experimental animals. Arch. Pharmacal Res. 2020, 43, 1335–1346. [Google Scholar] [CrossRef]
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018, 84, 139–152. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef]
- Han, M.; Hou, J.; Dong, C.; Li, W.; Yu, H.; Zheng, Y.; Chen, L. Isolation, Synthesis and Structures of Ginsenoside Derivatives and Their Anti-Tumor Bioactivity. Molecules 2010, 15, 399–406. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yang, S.-C.; Park, J.-K.; Jung, M.-W.; Lee, C.-J. Reduction of Electrically Evoked Neural Activity by Ginseng Saponin in Rat Hippocampal Slices. Biol. Pharm. Bull. 2000, 23, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shi, Z.; Dong, L.; Lv, J.; Xu, P.; Li, Y.; Qu, L.; Liu, X. Exploring the Effect of Ginsenoside Rh1 in a Sleep Deprivation-Induced Mouse Memory Impairment Model. Phytother. Res. 2017, 31, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Chu, S.; Wang, Y.; Wang, X.; Chen, N.; Zhang, J. Improvement of Memory in Mice and Increase of Hippocampal Excitability in Rats by Ginsenoside Rg1’s Metabolites Ginsenoside Rh1 and Protopanaxatriol. J. Pharmacol. Sci. 2009, 109, 504–510. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Lee, M.; Yu, J.; Sung, C. Long-term administration of ginsenoside Rh1 enhances learning and memory by promoting cell survival in the mouse hippocampus. Int. J. Mol. Med. 2014, 33, 234–240. [Google Scholar] [CrossRef]
- Gong, C.-X.; Singh, T.J.; Grundke-Iqbal, I.; Iqbal, K. Phosphoprotein Phosphatase Activities in Alzheimer Disease Brain. J. Neurochem. 1993, 61, 921–927. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Park, M.; Kim, S.-H.; Lee, H.-J. Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-β Induced SH-SY5Y Cells. Appl. Sci. 2021, 11, 5654. [Google Scholar] [CrossRef]
- Song, L.; Yao, L.; Zhang, L.; Piao, Z.; Lu, Y. Schizandrol A protects against Aβ1–42-induced autophagy via activation of PI3K/AKT/mTOR pathway in SH-SY5Y cells and primary hippocampal neurons. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1739–1752. [Google Scholar] [CrossRef]
- Yun-Feng, B.; Hang, S.; Ming-Zhu, Z.; Jing-Sheng, L. Effect of Ginsenoside Rh1 on Cognitive Impairment in Mice. Sci. Technol. Food Ind. 2019, 24, 300–304. [Google Scholar]
- Lozon, L.; Saleh, E.; Menon, V.; Ramadan, W.S.; Amin, A.; El-Awady, R. Effect of safranal on the response of cancer cells to topoisomerase I inhibitors: Does sequence matter? Front. Pharmacol. 2022, 13, 938471. [Google Scholar] [CrossRef] [PubMed]
- Abdu, S.; Juaid, N.; Amin, A.; Moulay, M.; Miled, N. Therapeutic Effects of Crocin Alone or in Combination with Sorafenib against Hepatocellular Carcinoma: In Vivo & In Vitro Insights. Antioxidants 2022, 11, 1645. [Google Scholar]
- Awad, B.; Hamza, A.A.; Al-Maktoum, A.; Al-Salam, S.; Amin, A. Combining Crocin and Sorafenib Improves Their Tumor-Inhibiting Effects in a Rat Model of Diethylnitrosamine-Induced Cirrhotic-Hepatocellular Carcinoma. Cancers 2023, 15, 4063. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Mohamed, M.G.; Lashin, F.M.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 81, 43. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Jin, Y.; Zhang, Q.; Li, R. Peripheral nerve repair: A hot spot analysis on treatment methods from 2010 to 2014. Neural Regen. Res. 2015, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, T.; Li, W.; Li, J.; Wang, C.; Zhang, K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol. Biol. Rep. 2021, 48, 2639–2652. [Google Scholar] [CrossRef]
- Yi, Y.-S. Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Ginseng Res. 2023, in press. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Al-Maktoum, A.; Amin, A. Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers 2023, 15, 3900. [Google Scholar] [CrossRef] [PubMed]
- Sarhene, M.; Ni, J.Y.; Duncan, E.S.; Liu, Z.; Li, S.; Zhang, J.; Guo, R.; Gao, S.; Gao, X.; Fan, G. Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol. Res. 2021, 166, 105481. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Rep. 2015, 11, 4518–4524. [Google Scholar] [CrossRef]
- Abdel Salam, O.M.E.; Sleem, A.A.; Shafee, N. Effect of Crataegus extract on carbon tetrachloride-induced hepatic damage. Comp. Clin. Pathol. 2012, 21, 1719–1726. [Google Scholar] [CrossRef]
- Noorbala, A.A.; Akhondzadeh, S. Attention-deficit/hyperactivity disorder: Etiology and pharmacotherapy. Arch. Iran. Med. 2006, 9, 374–380. [Google Scholar] [PubMed]
- Chen, J.; Zhang, H.; Yang, Y.; Chen, B. Quercetin regulates vascular endothelium function in chronic renal failure via modulation of Eph/Cav-1 signaling. Drug Dev. Res. 2022, 83, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Auti, A.; Alessio, N.; Ballini, A.; Dioguardi, M.; Cantore, S.; Scacco, S.; Vitiello, A.; Quagliuolo, L.; Rinaldi, B.; Santacroce, L.; et al. Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress. J. Pers. Med. 2022, 12, 1202. [Google Scholar] [CrossRef]
- Borges, S.C.; da Silva de Souza, A.C.; Beraldi, E.J.; Schneider, L.C.L.; Buttow, N.C. Resveratrol promotes myenteric neuroprotection in the ileum of rats after ischemia-reperfusion injury. Life Sci. 2016, 166, 54–59. [Google Scholar] [CrossRef]
- Kazemirad, H.; Kazerani, H.R. Cardioprotective effects of resveratrol following myocardial ischemia and reperfusion. Mol. Biol. Rep. 2020, 47, 5843–5850. [Google Scholar] [CrossRef]
- Salla, M.; Pandya, V.; Bhullar, K.S.; Kerek, E.; Wong, Y.F.; Losch, R.; Ou, J.; Aldawsari, F.S.; Martinez, C.V.; Thiesen, A.; et al. Resveratrol and Resveratrol-Aspirin Hybrid Compounds as Potent Intestinal Anti-Inflammatory and Anti-Tumor Drugs. Molecules 2020, 25, 3849. [Google Scholar] [CrossRef]
- Hamza, A.A.; Heeba, G.H.; Hassanin, S.O.; Elwy, H.M.; Bekhit, A.A.; Amin, A. Hibiscus-cisplatin combination treatment decreases liver toxicity in rats while increasing toxicity in lung cancer cells via oxidative stress- apoptosis pathway. Biomed. Pharmacother. 2023, 165, 115148. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Ren, Y.; Wu, X.; Liu, Y.; Wang, T.; Li, Y.; Cong, Y.; Guo, Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother. Res. 2021, 35, 5767–5780. [Google Scholar] [CrossRef]
- Dumurgier, J.; Tzourio, C. Epidemiology of neurological diseases in older adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Li, H.; Xia, W.; Liu, Q.; Zhou, X.; Dong, L.; Fu, X. Update on new trend and progress of the mechanism of polysaccharides in the intervention of Alzheimer’s disease, based on the new understanding of relevant theories: A review. Int. J. Biol. Macromol. 2022, 218, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Manju Bharadvaja, N. Exploring the Potential Therapeutic Approach Using Ginsenosides for the Management of Neurodegenerative Disorders. Mol. Biotechnol. 2023, 1–17. [Google Scholar]
- Ding, B.; Xie, C.; Xie, J.; Gao, Z.; Fei, X.; Hong, E.; Chen, W.; Chen, Y. Knockdown of NADPH oxidase 4 reduces mitochondrial oxidative stress and neuronal pyroptosis following intracerebral hemorrhage. Neural Regen. Res. 2023, 18, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Y.; Lu, M.-H.; Yuan, D.-J.; Xu, D.-E.; Yao, P.-P.; Ji, W.-L.; Chen, H.; Liu, W.; Yan, C.; Xia, Y.; et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Reeb, K.L.; Mortensen, O.V.; Fontana, A.C.K. Modulation of glutamate transporters as a potential therapeutic intervention for neurological disorders. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Angelova, P.R.; Vinogradova, D.; Neganova, M.E.; Serkova, T.P.; Sokolov, V.V.; Bachurin, S.O.; Shevtsova, E.F.; Abramov, A.Y. Pharmacological Sequestration of Mitochondrial Calcium Uptake Protects Neurons against Glutamate Excitotoxicity. Mol. Neurobiol. 2019, 56, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ondrejcak, T.; Yu, P.; Zhang, Y.; Yang, Y.; Klyubin, I.; Kennelly, S.P.; Rowan, M.J.; Hu, N. Do tau-synaptic long-term depression interactions in the hippocampus play a pivotal role in the progression of Alzheimer’s disease? Neural Regen. Res. 2023, 18, 1213. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dey, A.; Gopalakrishnan, A.V.; Swati, K.; Ojha, S.; Prakash, A.; Kumar, D.; Ambasta, R.K.; Jha, N.K.; Jha, S.K.; et al. Glutamatergic neurotransmission: A potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Res. Rev. 2023, 85, 101838. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.H.; Kim, Y.-J.; Kim, H.; Oh, Y.; Choi, K.Y.; Kim, B.C.; Lee, K.H.; Song, W.K. Elevation of phospholipase C-β1 expression by amyloid-β facilitates calcium overload in neuronal cells. Brain Res. 2022, 1788, 147924. [Google Scholar] [CrossRef]
- Lim Tung, H.Y. Phosphorylation of the calmodulin-dependent protein phosphatase by protein kinase C. Biochem. Biophys. Res. Commun. 1986, 138, 783–788. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Sun, K.; Liu, X.-Z.; Tong, K.-L.; Chen, Z.-J.; Yu, L.; Chen, N.; Liu, S. Inhibiting tau protein improves the recovery of spinal cord injury in rats by alleviating neuroinflammation and oxidative stress. Neural Regen. Res. 2023, 18, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Gao, Q.; Lin, J.; Chang, Z.; Wang, Y.; Shi, Y.; Su, R.; Han, Z.; Ma, D. Uncovering the mechanism of the Shenzhi Jiannao formula against vascular dementia using a combined network pharmacology approach and molecular biology. Phytomedicine 2021, 90, 153637. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Yang, L.; Zhang, D.; Li, L.; Dong, X.; Li, Y.; Qun, S.; Li, W. Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J. Ginseng Res. 2022, 46, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P. NMDA and AMPA receptors dysregulation in Alzheimer’s disease. Eur. J. Pharmacol. 2021, 908, 174310. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, R.; Zhang, R.; Xiao, C.; Wang, L.; Luo, M.; Song, N.; Zhang, J.; Yang, F.; Liu, X.; et al. Gastrodin and Gastrodigenin Improve Energy Metabolism Disorders and Mitochondrial Dysfunction to Antagonize Vascular Dementia. Molecules 2023, 28, 2598. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Xu, H.; Yu, X.; Lyu, C.; Tian, Y.; Guo, M.; Sun, J.; Sui, D. 20(S)-Ginsenoside Rg2 attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation: Role of SIRT1. RSC Adv. 2018, 8, 23947–23962. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, S.; Hou, L.; Wang, Y.; Xing, X.; Guan, T.; Zhang, J.; Li, T. Computational and experimental characterization of estrogenic activities of 20(S, R)-protopanaxadiol and 20(S, R)-protopanaxatriol. J. Ginseng Res. 2020, 44, 690–696. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Chen, K.-C.; Zhou, Y.; Wei, H.; Qi, M.-H.; Wang, Z.; Zheng, Y.; Chen, R.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomedicine 2022, 104, 154341. [Google Scholar] [CrossRef]
- Asanad, S.; Fantini, M.; Sultan, W.; Pogoda, J.M.; Nassisi, M.; Felix, C.; Wu, J.; Karanjia, R.; Cisneros, F.N.C.; Sadun, A.A.; et al. Retinal Nerve Fiber Layer Thinning In Pre-Clinical Alzheimer’s Disease Predicts Csf Amyloid/Tau Classification. Alzheimers Dement. 2019, 15, 1621–1622. [Google Scholar] [CrossRef]
- Tang, J.; Oliveros, A.; Jang, M.-H. Dysfunctional Mitochondrial Bioenergetics and Synaptic Degeneration in Alzheimer Disease. Int. Neurourol. J. 2019, 23 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, T.M.; Berkiks, I.; Pillay, S.; Scibiorek, M.; Moses, B.O.; Brombacher, F. IL-4R alpha deficiency influences hippocampal-BDNF signaling pathway to impair reference memory. Sci. Rep. 2020, 10, 16506. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Karen, S.; Beck, J.; Brand, S.; Hemmeter, U.; Hatzinger, M.; Trachsler, E.H. The Link Between Sleep, Stress and BDNF. Eur. Psychiatry 2017, 41, S282. [Google Scholar] [CrossRef]
- Taylor, N.E.; Ferrari, L. Discovering chronic pain treatments: Better animal models might help us get there. J. Clin. Investig. 2023, 133, e167814. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; Mayer, I.; Kammer, T.; Minkova, L.; Lahr, J.; Klöppel, S.; Grothe, M.J.; Orth, M. The relationship between cholinergic system brain structure and function in healthy adults and patients with mild cognitive impairment. Sci. Rep. 2021, 11, 16080. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Berardelli, I.; Serafini, G.; Cortese, N.; Fiaschè, F.; O’Connor, R.C.; Pompili, M. The Involvement of Hypothalamus–Pituitary–Adrenal (HPA) Axis in Suicide Risk. Brain Sci. 2020, 10, 653. [Google Scholar] [CrossRef]
- Dell’Oste, V.; Fantasia, S.; Gravina, D.; Palego, L.; Betti, L.; Dell’Osso, L.; Giannaccini, G.; Carmassi, C. Metabolic and Inflammatory Response in Post-Traumatic Stress Disorder (PTSD): A Systematic Review on Peripheral Neuroimmune Biomarkers. Int. J. Environ. Res. Public Health 2023, 20, 2937. [Google Scholar] [CrossRef]
- Shao, S.; Wang, G.L.; Raymond, C.; Deng, X.H.; Zhu, X.L.; Wang, D.; Hong, L. Activation of Sonic hedgehog signal by Purmorphamine, in a mouse model of Parkinson’s disease, protects dopaminergic neurons and attenuates inflammatory response by mediating PI3K/AKt signaling pathway. Mol. Med. Rep. 2017, 16, 1269–1277. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Xia, T.; Yang, X.; Sun, G.; Zhao, C.; Xu, C.; Zhang, H. Evolution of toll-like receptor gene family in amphibians. Int. J. Biol. Macromol. 2022, 208, 463–474. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, Y.; Chen, C.; Li, Z.; Jia, Y.; Su, X.; Wang, C.; He, X. Electroacupuncture Improved Hippocampal Neurogenesis following Traumatic Brain Injury in Mice through Inhibition of TLR4 Signaling Pathway. Stem Cells Int. 2017, 2017, 5841814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dong, H.; Lv, T.; Jin, K.; Jin, Y.; Zhang, X.; Jiang, J. Moderate hypothermia inhibits microglial activation after traumatic brain injury by modulating autophagy/apoptosis and the MyD88-dependent TLR4 signaling pathway. J. Neuroinflammation 2018, 15, 273. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J. NF-kB (p50/p65)-Mediated Pro-Inflammatory microRNA (miRNA) Signaling in Alzheimer’s Disease (AD). Front. Mol. Neurosci. 2022, 15, 943492. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Wang, Y.; Zang, C.; Liu, H.; Yuan, F.; Ning, J.; Shang, M.; Ma, J.; Li, G.; Yang, Y.; et al. Inhibition of Dyrk1A Attenuates LPS-Induced Neuroinflammation via the TLR4/NF-κB P65 Signaling Pathway. Inflammation 2022, 45, 2375–2387. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, W.; Zhao, Y.; Zong, Y.; Li, J.; He, Z. Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms. Molecules 2023, 28, 7935. https://doi.org/10.3390/molecules28237935
Liu S, Chen W, Zhao Y, Zong Y, Li J, He Z. Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms. Molecules. 2023; 28(23):7935. https://doi.org/10.3390/molecules28237935
Chicago/Turabian StyleLiu, Silu, Weijia Chen, Yan Zhao, Ying Zong, Jianming Li, and Zhongmei He. 2023. "Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms" Molecules 28, no. 23: 7935. https://doi.org/10.3390/molecules28237935
APA StyleLiu, S., Chen, W., Zhao, Y., Zong, Y., Li, J., & He, Z. (2023). Research Progress on Effects of Ginsenoside Rg2 and Rh1 on Nervous System and Related Mechanisms. Molecules, 28(23), 7935. https://doi.org/10.3390/molecules28237935