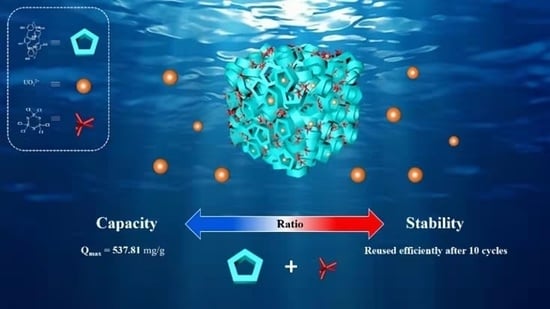
Synthesis of Pillar[5]arene- and Phosphazene-Linked Porous Organic Polymers for Highly Efficient Adsorption of Uranium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Adsorption Experiments
2.2.1. Effect of Acidity
2.2.2. Effect of Sorption Time and Kinetic Studies
2.2.3. Effect of Initial Concentration and Isotherm Studies
2.2.4. Mechanism of Uranium Sorption
2.2.5. The Recyclability of the HCCP-P5-2
2.2.6. The Uranium Sorption Proficiencies of Other Reported POPs
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Polymer HCCP-P5-1 and HCCP-P5-2
3.3. Materials Characterizations
3.4. Sorption Experiments
3.4.1. Uranium Sorption Isotherms
3.4.2. Uranium Adsorption Kinetics from U-Spiked Pure Water
3.4.3. Uranium Adsorption Kinetics from U-Spiked Simulated Seawater
3.4.4. The Recyclability of the Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xie, X.Q.; Wang, Y.F.; Xiong, Z.; Li, H.Z.; Yao, C. Highly efficient removal of uranium (VI) from aqueous solution using poly (cyclotriphosphazene-co-polyethyleneimine) microspheres. J. Radioanal. Nucl. Chem. 2020, 326, 1867–1877. [Google Scholar] [CrossRef]
- Singhal, R.K.; Basu, H.; Pimple, M.V.; Manisha, V.; Basan, M.K.T.; Reddy, A.V.R. Spectroscopic determination of U (VI) species sorbed by the Chlorella (Chlorella pyrenoidosa) freshwater algae. J. Radioanal. Nucl. Chem. 2013, 298, 587–592. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhao, Z.P.; Yuan, D.Z.; Wang, Y.; Dai, Y.; Zhu, Y.; Chew, J.W. Introduction of amino groups into polyphosphazene framework supported on CNT and coated Fe3O4 nanoparticles for enhanced selective U (VI) adsorption. Appl. Surf. Sci. 2019, 466, 893–902. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Yu, Q.H.; Wen, J.; Li, C.Y.; Guo, Z.H.; Wang, X.L.; Wang, N. Ultrafast and Highly Selective Uranium Extraction from Seawater by Hydrogel-like Spidroin-based Protein Fiber. Angew. Chem. Int. Ed. 2019, 58, 11785–11790. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.M.; Yan, Q.; Wang, M.X.; Chen, J.; Fu, H.Y.; Ye, J.W.; Conradson, S.D.; Yuan, L.H.; Xu, C.; Feng, W. Endowing 2,6-bis-triazolyl-pyridine of poor extraction with superior efficiency for actinide/lanthanide separation at high acidity by anchoring to a macrocyclic scaffold. J. Hazard. Mater. 2021, 416, 125745. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, Z.; Lu, Y.X.; Liu, S.; Li, H.P.; Ye, G.; Chen, J. Graphene Oxide Membranes for Tunable Ion Sieving in Acidic Radioactive Waste. Adv. Sci. 2021, 8, 2002717–2002724. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, M.K.; Das, D.; Mallia, M.B.; Gupta, P.C. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J. Hazard. Mater. 2010, 184, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhang, D.X.; Xu, X.Y.; Reda, A.T.; Li, J.Y. Efficient electrosorption of uranyl ions by a homemade amidoxime-modified carbon paper-based electrode in acidic aqueous condition. J. Chem. Technol. Biotechnol. 2021, 96, 2916–2929. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, X.S.; Li, B.; Bai, C.Y.; Li, Y.; Wang, L.; Wen, R.; Zhang, M.; Ma, L.; Li, S. “Stereoscopic” 2D super-microporous phosphazene-based covalent organic framework: Design, synthesis and selective sorption towards uranium at high acidic condition. J. Hazard. Mater. 2016, 314, 95–104. [Google Scholar] [CrossRef]
- Zhang, M.C.; Li, Y.; Bai, C.Y.; Guo, X.H.; Han, J.; Hu, S.; Jiang, H.; Tan, W.; Li, S.J.; Ma, L.J. Synthesis of Microporous Covalent Phosphazene-Based Frameworks for Selective Separation of Uranium in Highly Acidic Media Based on Size-Matching Effect. ACS Appl. Mater. Interfaces 2018, 10, 28936–28947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, Y.F.; Yao, C. Highly efficient removal of uranium (VI) from aqueous solution using the Chitosan- Hexachlorocyclotriphosphazene composite. J. Radioanal. Nucl. Chem. 2021, 330, 113–125. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Thallapally, P.K.; Ma, S. Covalent Organic Frameworks as a Decorating Platform for Utilization and Affinity Enhancement of Chelating Sites for Radionuclide Sequestration. Adv. Mater. 2018, 30, 1705479–1705487. [Google Scholar] [CrossRef] [PubMed]
- Chouyyok, W.; Pittman, J.W.; Warner, M.G.; Nell, K.M.; Clubb, D.C.; Gill, G.A.; Addleman, R.S. Surface functionalized nanostructured ceramic sorbents for the effective collection and recovery of uranium from seawater. Dalton Trans. 2016, 45, 11312–11325. [Google Scholar] [CrossRef] [PubMed]
- Li, R.M.; Che, R.; Liu, Q.; Su, S.Z.; Li, Z.S.; Zhang, H.S.; Liu, J.J.; Liu, L.H.; Wang, J. Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J. Hazard. Mater. 2017, 338, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.Y.; Wang, Z.; Zhang, Z.J.; Wang, X.D.; Yang, Z.L. Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J. Alloys Compd. 2021, 852, 156993. [Google Scholar] [CrossRef]
- Hu, B.W.; Wang, H.F.; Liu, R.R.; Qiu, M.Q. Highly efficient U (VI) capture by amidoxime/carbon nitride composites: Evidence of EXAFS and modeling. Chemosphere 2021, 274, 129743–129751. [Google Scholar] [CrossRef]
- Tripathi, A.; Melo, J.S. Synthesis of low-density biopolymeric chitosan–agarose cryomatrix and its surface functionalization with bio-transformed melanin for the enhanced recovery of uranium (vi) from aqueous subsurfaces. RSC Adv. 2016, 6, 37067–37078. [Google Scholar] [CrossRef]
- Yang, P.P.; Liu, Q.; Liu, J.Y.; Zhang, H.S.; Li, Z.S.; Li, R.M.; Liu, L.H.; Wang, J. Interfacial growth of a metal–organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium (vi). J. Mater. Chem. A 2017, 5, 17933–17942. [Google Scholar] [CrossRef]
- Zhong, X.; Wen, L.; Wang, H.F.; Xue, C.; Hu, B.W. Aluminum-based metal-organic frameworks (CAU-1) highly efficient UO2(2+) and TcO4(-) ions immobilization from aqueous solution. J. Hazard. Mater. 2021, 407, 124729. [Google Scholar] [CrossRef]
- Feng, L.J.; Wang, H.; Feng, T.T.; Yan, B.J.; Yu, Q.H.; Zhang, J.C.; Guo, Z.H.; Yuan, Y.H.; Ma, C.X.; Liu, T.; et al. In-situ synthesis of uranyl-imprinted nanocage for selective uranium recovery from seawater. Angew. Chem. Int. Ed. 2021, 61, 82–86. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Gong, W.T.; Deng, X.R.; Dong, K.X.; Liu, L.; Ning, G.L. A boranil-based conjugated microporous polymer for efficient visible-light-driven heterogeneous photocatalysis. Polym. Chem. 2021, 12, 3153–3159. [Google Scholar] [CrossRef]
- Gong, W.T.; Dong, K.X.; Liu, L.; Hassan, M.; Ning, G.L. β-Diketone boron difluoride dye-functionalized conjugated microporous polymers for efficient aerobic oxidative photocatalysis. Catal. Sci. Technol. 2021, 11, 3905–3913. [Google Scholar] [CrossRef]
- Qu, W.D.; Zhang, S.G.; Dong, K.X.; Deng, X.R.; Gong, W.T.; Ning, G.L. Construction of rigid ionic porous organic polymers (iPOPs) via Zincke reaction with tunable absorption behaviors. J. Porous Mater. 2021, 28, 507–514. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Gong, W.T.; Qu, W.D.; Deng, X.R.; Dong, K.X.; Zhang, S.G.; Ning, G.L. Construction of Ionic Porous Organic Polymers (iPOPs) via Pyrylium Mediated Transformation. Chin. J. Polym. Sci. 2020, 38, 958–964. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, T.; Gao, P.; Zhao, L.; Zhou, L.; Hua, D.B. Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J. Mater. Chem. A 2019, 7, 11214–11222. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, L.J.; Ma, F.Q.; Zhu, X.Y.; Dong, H.X.; Zhang, C.H.; Zhao, F.B. Synthesis of phosphorylated hyper-cross-linked polymers and their efficient uranium adsorption in water. J. Hazard. Mater. 2021, 419, 126538. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, H.; Lin, M.Z.; Wen, J.; Hu, S. Effects of chain conformation on uranium adsorption performance of amidoxime adsorbents. Sep. Purif. Technol. 2023, 307, 122777. [Google Scholar] [CrossRef]
- Sakr, A.K.; Al-Hamarneh, I.F.; Gomaa, H.; Abdel Aal, M.M.; Hanfi, M.Y.; Sayyed, M.I.; Khandaler, S.U.; Cheira, M.F. Re-moval of uranium from nuclear effluent using regenerated bleaching earth steeped in β–naphthol. Radiat. Phys. Chem. 2022, 200, 110204. [Google Scholar] [CrossRef]
- Atia, B.M.; Sakr, A.K.; Gado, M.A.; El-Gendy, H.S.; Abdelazeem, N.M.; El-Sheikh, E.M.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Synthesis of a New Chelating Iminophosphorane Derivative (Phosphazene) for U (VI) Recovery. Polymers 2022, 14, 1687. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Gong, W.T.; Hassan, M.; Qu, W.D.; Liu, L.; Ning, G.L. Guest induced morphology transitions of star shaped pillar[5]arene trimer via endo host-guest and “exo-wall” electron-transfer interactions. Chin. Chem. Lett. 2021, 32, 371–374. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.M.; Huang, S.F.; Jin, Y.H.; Cui, J.N.; Gong, W.T.; Zhang, W. A pillar[5]arene-based covalent organic framework with pre-encoded selective host–guest recognition. Chem. Sci. 2021, 12, 13316–13320. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Yang, Z.Q.; Li, Z.; Wu, J.R.; Yang, B.; Yang, Y.W. Construction of Hydrazone-Linked Macrocycle-Enriched Covalent Organic Frameworks for Highly Efficient Photocatalysis. Chemistry of Materials. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Z.Q.; Zhang, Y.N.; Yang, B.Y.; Yang, Y.W. Synthesis of an Acidochromic and Nitroaromatic Responsive Hydrazone-Linked Pillararene Framework by a Macrocycle-To-Framework Strategy. Angew. Chem. Int. Ed. 2022, 61, e202206144. [Google Scholar] [CrossRef]
- Chen, W.B.; Chen, P.; Zhang, G.; Xing, G.L.; Feng, Y.; Yang, Y.W.; Chen, L. Macrocycle-derived hierarchical porous organic polymers: Synthesis and applications. Chem. Soc. Rev. 2021, 50, 11684–11714. [Google Scholar] [CrossRef]
- Li, M.H.; Lou, X.Y.; Yang, Y.W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021, 57, 13429–13447. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Yao, Y.; Huang, F.H. Four Pillar[5]arene Constitutional Isomers: Synthesis, Crystal Structures, and Host-Guest Complexation of Their Derivatives with Paraquat in Water. Chin. J. Chem. 2015, 33, 356–360. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, X.H.; Gong, W.T.; Sun, T.J.; Wang, Z.G.; Ning, G.L. Pillar[5]arene-Derived Microporous Polyaminal Networks with Enhanced Uptake Performance for CO2 and Iodine. Ind. Eng. Chem. Res. 2020, 59, 3269–3278. [Google Scholar] [CrossRef]
- Tan, L.L.; Li, H.; Tao, Y.; Zhang, S.X.; Wang, B.; Yang, Y.W. Pillar[5]arene-based supramolecular organic frameworks for highly selective CO2-capture at ambient conditions. Adv. Mater. 2014, 26, 7027–7031. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.Y.; Li, J.; Liu, S.B.; Yang, X.Y.; Yang, X.D.; Tian, Y.; Cao, K.C.; Huang, Y.; Ma, L.J.; Li, S.J. In situ preparation of nitrogen-rich and functional ultramicroporous carbonaceous COFs by “segregated” microwave irradiation. Microporous Mesoporous Mater. 2014, 197, 148–155. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zhang, M.C.; Li, B.; Zhao, X.S.; Zhang, S.; Wang, L.; Li, Y.; Zhang, J.; Ma, L.J.; Li, S.J. Modifiable diyne-based covalent organic framework: A versatile platform for in situ multipurpose functionalization. RSC Adv. 2016, 6, 39150–39158. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.D.; Bai, C.Y.; Tian, Y.; Li, B.; Zhang, S.; Yang, X.Y.; Ding, S.D.; Xia, C.Q.; Tan, X.Y.; et al. A novel benzimidazole-functionalized 2-D COF material: Synthesis and application as a selective solid-phase extractant for separation of uranium. J. Colloid Interface Sci. 2015, 437, 211–218. [Google Scholar] [CrossRef]
- Yu, J.P.; Yuan, L.Y.; Wang, S.; Lan, J.H.; Zheng, L.R.; Xu, C.; Chen, J.; Wang, L.; Huang, Z.W.; Tao, W.Q.; et al. Phosphonate-decorated covalent organic frameworks for actinide extraction: A breakthrough under highly acidic conditions. CCS Chem. 2019, 1, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Y.; Sun, Q.; Zhang, Y.M.; Abney, C.W.; Aguila, B.; Lin, W.B.; Ma, S.Q. Functionalized Porous Aromatic Framework for Efficient Uranium Adsorption from Aqueous Solutions. ACS Appl. Mater. Interfaces 2017, 9, 12511–12517. [Google Scholar] [CrossRef]
- Cui, W.R.; Zhang, C.R.; Jiang, W.; Li, F.F.; Liang, R.P.; Liu, J.; Qiu, J.D. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 2020, 11, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.H.; Yu, Z.W.; Gong, L.L.; Tao, Y.; Gao, Z.; Wang, L.; Yin, W.H.; Yang, L.X.; Luo, F. Ammoniating Covalent Organic Framework (COF) for High-Performance and Selective Extraction of Toxic and Radioactive Uranium Ions. Adv. Sci. 2019, 6, 1900547–1900554. [Google Scholar] [CrossRef] [Green Version]
- You, Z.X.; Zhang, N.; Guan, Q.L.; Xing, Y.H.; Bai, F.Y.; Sun, L.X. High Sorption Capacity of U (VI) by COF-Based Material Doping Hydroxyapatite Microspheres: Kinetic, Equilibrium and Mechanism Investigation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1966–1979. [Google Scholar] [CrossRef]









| POPs | SBET (m2/g) | VTotal (cc/g) a | Pore Size (nm) |
|---|---|---|---|
| HCCP-P5-1 | 17.1 | 0.043 | 4.728 |
| HCCP-P5-2 | 23.3 | 0.058 | 4.728 |
| Solution | Polymers | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| Qe (mg/g) | K1 | R2 | Qe (mg/g) | K2 | R2 | ||
| Pure water | HCCP-P5-1 | 310.23 | 0.037 | 0.810 | 401.60 | 4.95 × 10−5 | 0.995 |
| HCCP-P5-2 | 207.64 | 0.047 | 0.821 | 271.73 | 7.50 × 10−5 | 0.997 | |
| Simulated seawater | HCCP-P5-1 | 279.37 | 0.064 | 0.872 | 335.57 | 1.09 × 10−4 | 0.999 |
| HCCP-P5-2 | 92.27 | 0.062 | 0.930 | 107.64 | 3.76 × 10−4 | 0.999 | |
| Polymers | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL | R2 | KF | N | R2 | |
| HCCP-P5-1 | 537.81 | 0.0986 | 0.976 | 170.28 | 0.245 | 0.793 |
| HCCP-P5-2 | 473.32 | 0.0696 | 0.973 | 128.81 | 0.274 | 0.799 |
| No | Adsorption | qm (mg/g) | pH | Ref. |
|---|---|---|---|---|
| 1 | CCOF-SCU1 | 50 | 1.0 | [43] |
| 2 | COF TCD | 158 | 4.5 | [44] |
| 3 | MPCOF | 214 | 4.5 | [10] |
| 4 | COF-TpAb-AO | 408 | 6.0 | [13] |
| 5 | COF-HBI | 211 | 4.5 | [45] |
| 6 | COF-IHEP1 | 112 | 5.0 | [46] |
| 7 | PAF-1-CH2AO | 300 | —— | [47] |
| 8 | TFPT-BTAN-AO | 427 | 4.0 | [48] |
| 9 | COF-SO3H | 360 | 5.0 | [49] |
| 10 | COF-HAP | 510 | 3.0 | [50] |
| 11 | HCCP-P5-1 | 537 | 6.0 | This work |
| 12 | HCCP-P5-2 | 473 | 6.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, Z.; Zhang, S.; Hassan, M.; Ma, C.; Liu, Z.; Gong, W. Synthesis of Pillar[5]arene- and Phosphazene-Linked Porous Organic Polymers for Highly Efficient Adsorption of Uranium. Molecules 2023, 28, 1029. https://doi.org/10.3390/molecules28031029
Zhao X, Liu Z, Zhang S, Hassan M, Ma C, Liu Z, Gong W. Synthesis of Pillar[5]arene- and Phosphazene-Linked Porous Organic Polymers for Highly Efficient Adsorption of Uranium. Molecules. 2023; 28(3):1029. https://doi.org/10.3390/molecules28031029
Chicago/Turabian StyleZhao, Xiaoxiao, Ziyi Liu, Shuguang Zhang, Mehdi Hassan, Chunxin Ma, Zhenzhong Liu, and Weitao Gong. 2023. "Synthesis of Pillar[5]arene- and Phosphazene-Linked Porous Organic Polymers for Highly Efficient Adsorption of Uranium" Molecules 28, no. 3: 1029. https://doi.org/10.3390/molecules28031029
APA StyleZhao, X., Liu, Z., Zhang, S., Hassan, M., Ma, C., Liu, Z., & Gong, W. (2023). Synthesis of Pillar[5]arene- and Phosphazene-Linked Porous Organic Polymers for Highly Efficient Adsorption of Uranium. Molecules, 28(3), 1029. https://doi.org/10.3390/molecules28031029