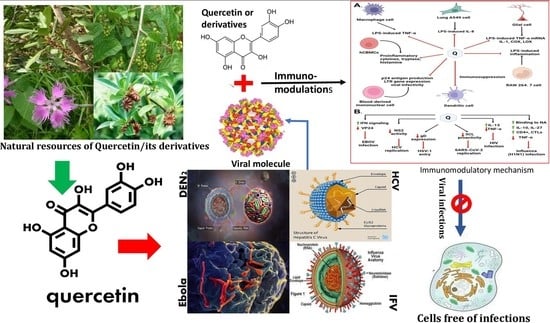
Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions
Abstract
:1. Introduction
2. Methodology and Resources
3. Research Question and Hypothesis
4. Natural Sources of Quercetin and Its Isolation from Plants
| Phytochemical | Plant Name | Family | Plant Parts | Virus Target | Cell | Bioassay | Viral Step or MOA | Reference |
|---|---|---|---|---|---|---|---|---|
| Quercetin-3-o-α-L-rhamnopyranoside (Q3R) | Rapanea melanophloeos | Myrsinaceae | Whole plant | IAV | MDCK cell | In vitro | Inhibit viral entry and virus replication | [41] |
| Quercetin 3-glucoside | Dianthus superbus L. | Caryophyllaceae | Whole plant | IAV | MDCK cell | In vitro and in silico | Inhibit viral replication | [42] |
| Quercitrin (Quercetin-3-L-rhamnoside) | Houttuynia cordata Thunb. | Saururaceae | Leaf (Aerial parts) | IAV (Anti-influenza A/WS/33 virus) | Mammalian kidney (BHK) | In vitro | Inhibit replication in the initial stage of virus infection by indirect interaction with virus particles | [43] |
| Rutin (Quercetin-3-rutinoside) | Prunus domestica | Rosaceae | Fruit | HCV | Human hepatocellular carcinoma cells Huh 7 and Huh 7.5 | In vitro and ex vivo | Inhibit the early stage of viral entry | [44] |
| Quercetin | Psidium guajava | Myrtaceae | Bark | DENV | Epithelial VERO cells (Cercopithecus aethiops) | In vitro and in silico | Directly inhibit the viral NS3 protein and could interrupt virus entry by inhibiting fusion | [45] |
| Quercetin | Embelia ribes | Myrsinaceae | Seeds | HCV | Huh-7 cells | In vitro | Inhibit NS3 protease activity and HCV replication. | [16] |
| Quercetin 7-rhamnoside | Houttuynia cordata | Saururaceae | Aerial Parts | Porcine epidemic diarrhea virus (PEDV CV 777) | Vero (african green monkey kidney cell line; ATCC CCR-81) ST (pig testis cell line; ATCC CRL-1746) | In vitro and In vivo | Inhibit at an early stage of viral replication after infection | [29] |
| Quercetin and its glycoside derivatives | Bauhinia longifolia (Bong.) | Fabaceae | Leaves | Mayaro viruses (ATCC VR-66, lineage TR 4675) | Vero cells (African green monkey kidney, ATCC CCL-81) | In vitro | glycosilation re duces the antiviral activity of Quercetin against | [12] |
| DihydroQuercetin (DHQ) | Larix sibirica (larch wood) | Pinaceae | Wood | Coxsackie virus B4 Powers strain | Vero cells Inbred, female mice | In vivo | Decrease the replication of viral protein by reducing ROS generation | [46] |
| Quercetin-7-o-glucoside | Dianthus superbus | Caryophyllaceae | Leaves | Influenz viruses A/Vic/3/75 (H3N2, VR-822), A/PR/8/34 (H1N1, VR-1469), B/Maryland/1/59 (VR-296) and B/Lee/40 (VR-1535D) | Madin-Darby Canine Kidney (MDCK) cell | In Vitro | Inhibit influenza viral RNA polymerase PB2 | [24] |
| Quercetin and Isoquercitrin | Houttuynia cordata | Saururaceae | Whole plant | Herpes simplex virus (HSV) | African green monkey kidney cells (Vero, ATCC CCL-81) and human epithelial carcinoma cells | In vitro | Quercetin and isoquercitrin inhibit NF-κB activation in HSV viral replication | [47] |
| Kaempferol | Rhodioila rosea | Crassulaceae | Roots | The influenza strains A/PR/8/34 (H1N1) (ATCC VR-1469) | Madin-Darby canine kidney (MDCK) cells were obtained | In vitro | Inhibit viral replication by blocking neuraminidases | [48] |
| Myricetin | Marcetia taxifolia | Melastomataceae | Aerial parts | HIV-1 (HTLV-IIIB/H9) | MT4 cells | In silico | May Bind to NNRTI pocket of NNRTI resistant HIV-1 | [49] |
| Apigein | Gentiana veitchiorum | Gentianaceae | Flower | Foot-and-mouth disease virus (FMDV) | BHK-21 cells | In vitro | Block the internal ribosome entry site (IRES) mediate translational activity | [50,51] |
| Quercetin 3-o-β-glucopyranoside | Morus Alba | Moraceae | Leaf | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chair termination | [52] |
| Quercetin 3-o-β-(6”-o-galloyl)-glucopyranoside | Morus Alba | Moraceae | Leaf | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chair termination | [52] |
| Quercetin-3-o-β-L-rhamnopyranosyl | Acacia albdai | Fabaceae | Leaf | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chain termination | [52] |
| Quercetin-3-O-α-L-rhamnopyranoside | Acacia albdai | Fabaceae | Leaf | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chain termination | [52] |
| 6-o-methoxy Quercetin-7-o-β-D-glucopyranoside | Centaurea glomerata | Asteraceae | Aerial parts | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chain termination | [52] |
| 4’,6-o-dimethoxy Quercetin-7-o-β-D-glucopyranoside | Centaurea glomerata | Asteraceae | Areal Parts | Herpes simplex Virus type 1 | Vero cell line no ATCC CCL-81) | In vitro | Inhibit DNA chain termination | [52] |
| Quercetin-3-β-o-D-glucoside | Allium cepa | Amaryllidaceae | Root | Ebolaviruses (EBOV-Kikwit-GFP, EBOV Makona, SUDV-Boniface, mouse-adapted EBOV) | Vero E6 cells | In vitro | Block glycoprotein mediated step during viral entry | [53,54] |
| Isorhamnetin | Ginkgo biloba | Ginkgoaceae | Leaf | Influenza A virus Puerto Rico/8/34 (H1N1) | Madin Darby Canine Kidney (MDCK) cells | In vitro and In vivo | Inhibit neuraminidase and hemagglutination, suppress ROS generation and ERK phosphorylation | [55,56] |
| Luteolin | Elsholtzia rugulosa | Lamiaceae | Whole Plant | Influenza viruses A/PR/8/34(H1N1), A/Jinan/15/90(H3N2) and B/ Jiangsu/10/2003 | MDCK cells | In vitro | Inhibit the neuraminidase | [57] |
| Luteolin | Cynodon dactylon | Poaceae | Whole Plant | Chikungunya virus | Vero cells | In vitro | Inhibit intracellular viral replication | [58] |
| Quercetin | Illicium verum | Schisandraceae | Singapore grouper iridovirus (SGIV) | Grouper spleen (GS) cells | In vitro | Interrupt SGIV binding to host cell by blocking membrane receptor on host cell which | [59] | |
| Naringenin | Citrus sinensis | Rutaceae | Fruit | Zika Virus | Human A549 cells | In vitro | Inhibit NS2B-NS3 protease | [60,61] |
| Hesperidin | Citrus sinensis (sweet orange) | Rutaceae | Fruit Peel | SARS-CoV-2 virus | In silico | Binds to main protease and angiotensin converting enzyme 2 | [62] | |
| Hesperidin | Citrus sinensis | Rutaceae | Fruit Peel | Sindbis virus | BHK-2 | In vitro | Inhibitory activity on viral replication | [63,64] |
| Naringenin | Citrus paradisi | Rutaceae | Fruit Peel | Hepatitis C virus (HCV) | Huh7.5.1 human hepatoma cell | In vitro and In vivo | inhibits ApoB lipoprotein reduce secretion of HCV | [65,66] |
| Luteolin | Achyrocline satureioides | Asteraceae | Whole Plant | Influenza virus A/Fort Monmouth/1/1947 (H1N1) | Madin-Darby canine kidney (MDCK) cells and Vero cells | In vitro | Block absorption to the cell surface or receptor binding site leads to the suppress of the expression of coat protein I | [67,68] |
| Naringenin | Citrus paradisi | Rutaceae | Fruit Peel | Dengue virus (DENV) | Huh7.5 cells | In vitro | Act as antiviral cytokine during DENV replication | [66,69] |
5. Absorption, Metabolism, Distribution, and Excretion of Quercetin
6. Major Pharmacological Actions of Quercetin
7. Antiviral Actions of Quercetin
7.1. Quercetin against Hepatitis C Virus (HCV)
7.2. Mechanism of Quercetin against HCV Virus
7.3. Quercetin against Dengue Virus-2 (DENV-2)
7.4. Mechanism of Quercetin against DENV-2 Virus
7.5. Quercetin against Ebola Virus (EBOV)
7.6. Mechanism of Quercetin against EBOV Virus
7.7. Quercetin against Influenza A Virus
7.8. Mechanism of Quercetin against Influenza A Virus
7.8.1. Inhibiting Influenza Virus Entry via Blocking of HA and NA
7.8.2. Inhibiting Influenza Virus via Blocking of Viral RNA Polymerase
8. Quercetin in Preventing Viral Infection through Immunomodulation
9. Research Insights and Future Use of Quercetin
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Campos, F.S.; de Arruda, L.B.; da Fonseca, F.G. Special Issue “Viral Infections in Developing Countries”. Viruses 2022, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Piaserico, S.; Bordin, C.; Alaibac, M.; Girolomoni, G.; Naldi, L. Cutaneous manifestations of SARS-CoV-2 infection: A clinical update. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2021, 36, 266–278. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Arumugaswami, V.; Raychaudhuri, S.; Yeh, G.K.; Maloney, E.M.; Wang, J.; Dasgupta, A.; French, S.W. Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology 2012, 433, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit Vectors 2014, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Narayanan, S.; Chang, K.-O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir. Res. 2010, 88, 227–235. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; VanLandingham, D.L.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Son, M.; Ryu, E.; Shin, Y.S.; Kim, J.G.; Kang, B.W.; Sung, G.-H.; Cho, H.; Kang, H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget 2015, 6, 12603–12624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral. Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [Green Version]
- Fanunza, E.; Iampietro, M.; Distinto, S.; Corona, A.; Quartu, M.; Maccioni, E.; Horvat, B.; Tramontano, E. Quercetin Blocks Ebola Virus Infection by Counteracting the VP24 Interferon-Inhibitory Function. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Rahman, A.; Shorobi, F.M.; Uddin, N.; Saha, S.; Hossain, A. Quercetin attenuates viral infections by interacting with target proteins and linked genes in chemicobiological models. Silico Pharmacol. 2022, 10, 17. [Google Scholar] [CrossRef]
- Verma, K.; Sahu, S.; Saha, S.; Bahadur, S.; Bhardwaj, S. Review on quercetin and their beneficial properties. World J. Pharm. Pharm. Sci. 2018, 7, 395–403. [Google Scholar] [CrossRef]
- Herrmann, K. Flavonols and flavones in food plants: A review. Int. J. Food Sci. Technol. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Lin, C.-F.; Leu, Y.-L.; Al-Suwayeh, S.A.; Ku, M.-C.; Hwang, T.-L.; Fang, J.-Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Fernandes, I.; González-Manzano, S.; de Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 2016, 23, 958–967. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharmacal Res. 2008, 31, 640–644. [Google Scholar] [CrossRef]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J. Basic Med. Sci. 2015, 18, 635–643. [Google Scholar] [CrossRef]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef]
- Ying, H.-Z.; Liu, Y.-H.; Yu, B.; Wang, Z.-Y.; Zang, J.-N.; Yu, C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2012, 52, 53–60. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.-J.; Claycombe, K.J.; Song, W.O. Serum C-Reactive Protein Concentrations Are Inversely Associated with Dietary Flavonoid Intake in U.S. Adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ebrahimi, S.N.; Fotouhi, F.; Eskandari, F.; Eloff, J.N.; McGaw, L.J.; Fasina, F.O. Experimental validation and computational modeling of anti-influenza effects of quercetin-3-O-α-L-rhamnopyranoside from indigenous south African medicinal plant Rapanea melanophloeos. BMC Complement. Altern. Med. 2019, 19, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2019, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Correspondence, A. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar] [CrossRef]
- Zahoor, M.; Shah, A.B.; Naz, S.; Ullah, R.; Bari, A.; Mahmood, H.M. Isolation of Quercetin from Rubus fruticosus, Their Concentration through NF/RO Membranes, and Recovery through Carbon Nanocomposite. A Pilot Plant Study. BioMed. Res. Int. 2020, 2020, 8216435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wianowska, D. Application of Sea Sand Disruption Method for HPLC Determination of Quercetin in Plants. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1037–1043. [Google Scholar] [CrossRef]
- Wianowska, D.; Dawidowicz, A.L.; Bernacik, K.; Typek, R. Determining the true content of quercetin and its derivatives in plants employing SSDM and LC–MS analysis. Eur. Food Res. Technol. 2017, 243, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Saraswathi, V.S.; Saravanan, D.; Santhakumar, K. Isolation of quercetin from the methanolic extract of Lagerstroemia speciosa by HPLC technique, its cytotoxicity against MCF-7 cells and photocatalytic activity. J. Photochem. Photobiol. B Biol. 2017, 171, 20–26. [Google Scholar] [CrossRef]
- Tsague, R.K.T.; Kenmogne, S.B.; Tchienou, G.E.D.; Parra, K.; Ngassoum, M.B. Sequential extraction of quercetin-3-O-rhamnoside from Piliostigma thonningii Schum. leaves using microwave technology. SN Appl. Sci. 2020, 2, 1–17. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Ji, H.; Deng, J.; Lu, P.; Jiang, Z.; Li, X.; Wang, Y.; Wang, C.; Zhao, J.; et al. Quercetin synergistically reactivates human immunodeficiency virus type 1 latency by activating nuclear factor-κB. Mol. Med. Rep. 2017, 17, 2501–2508. [Google Scholar] [CrossRef]
- Cao, X.; Wei, Y.; Ito, Y. Preparative Isolation of Isorhamnetin from Stigma Maydis using High Speed Countercurrent Chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 273–280. [Google Scholar] [CrossRef]
- Mehrbod, P.; Abdalla, M.A.; Fotouhi, F.; Heidarzadeh, M.; Aro, A.O.; Eloff, J.N.; McGaw, L.J.; Fasina, F.O. Immunomodulatory properties of quercetin-3-O-α-L-rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complement. Altern. Med. 2018, 18, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nile, S.H.; Kim, D.H.; Nile, A.; Park, G.S.; Gansukh, E.; Kai, G. Probing the effect of quercetin 3-glucoside from Dianthus superbus L against influenza virus infection- In vitro and in silico biochemical and toxicological screening. Food Chem. Toxicol. 2019, 135, 110985. [Google Scholar] [CrossRef]
- Chiow, K.; Phoon, M.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, M.; Kamra, M.; Mullick, R.; Bhattacharya, S.; Das, S.; Karande, A.A. Identification of a flavonoid isolated from plum (Prunus domestica) as a potent inhibitor of Hepatitis C virus entry. Sci. Rep. 2017, 7, 3965. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef]
- Hung, P.-Y.; Ho, B.-C.; Lee, S.-Y.; Chang, S.-Y.; Kao, C.-L.; Lee, S.-S.; Lee, C.-N. Houttuynia cordata Targets the Beginning Stage of Herpes Simplex Virus Infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorganic Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T.; Suárez, A.I.; Serrano, M.L.; Baptista, J.; Pujol, F.H.; Rangel, H.R. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res. Ther. 2017, 14, 57. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Zhou, Z.; Ren, R.; Xu, M. Apigenin, flavonoid component isolated from Gentiana veitchiorum flower suppresses the oxidative stress through LDLR-LCAT signaling pathway. Biomed. Pharmacother. 2020, 128, 110298. [Google Scholar] [CrossRef]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Toumy, S.A.; Salib, J.Y.; El Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β- O-d-Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [Green Version]
- Sati, P.; Dhyani, P.; Bhatt, I.D.; Pandey, A. Ginkgo biloba flavonoid glycosides in antimicrobial perspective with reference to extraction method. J. Tradit. Complement. Med. 2019, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral Effect of Methylated Flavonol Isorhamnetin against Influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, J.; Li, W.; Shen, L.; Huang, S.; Tang, J.; Duan, J.; Fang, F.; Huang, Y.; Chang, H.; et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016, 6, 19095. [Google Scholar] [CrossRef] [Green Version]
- Murali, K.S.; Sivasubramanian, S.; Vincent, S.; Murugan, S.B.; Giridaran, B.; Dinesh, S.; Gunasekaran, P.; Krishnasamy, K.; Sathishkumar, R. Anti—Chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015, 8, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.-L.; Liu, B.; Qin, H.-L.; Lee, S.; Wang, Y.-T.; Du, G.-H. Anti-Influenza Virus Activities of Flavonoids from the Medicinal Plant Elsholtzia rugulosa. Planta Med. 2008, 74, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Almuzafar, H.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.-S.; Abdelazeem, W.H. The Preventive Effects and the Mechanisms of Action of Navel Orange Peel Hydroethanolic Extract, Naringin, and Naringenin in N-Acetyl-p-aminophenol-Induced Liver Injury in Wistar Rats. Oxidative Med. Cell. Longev. 2019, 2019, 2745352. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.V., Jr.; et al. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Alzuru, M.; Mendez, J.; Rodríguez-Ortega, M. Anti-Sindbis Activity of Flavanones Hesperetin and Naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar] [CrossRef] [Green Version]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Nahmias, Y.; Goldwasser, J.; Casali, M.; van Poll, D.; Wakita, T.; Chung, R.T.; Yarmush, M.L. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008, 47, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Si-Si, W.; Liao, L.; Ling, Z.; Yun-Xia, Y. Inhibition of TNF-α/IFN-γ induced RANTES expression in HaCaT cell by naringin. Pharm. Biol. 2011, 49, 810–814. [Google Scholar] [CrossRef]
- Yan, H.; Ma, L.; Wang, H.; Wu, S.; Huang, H.; Gu, Z.; Jiang, J.; Li, Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019, 73, 487–496. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; das Graças Fernandes da Silva, M.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Duarte Dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Moon, J.H.; Nakata, R.; Oshima, S.; Inakuma, T.; Terao, J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R461–R467. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid. -Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.; Lin, H.; Yang, Y.; Zhang, S.; Wu, X.; Wang, M.; Ji, L.; Lu, L.; Yu, L.; Han, G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010, 10, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kashiwabara, M.; Asano, K. Inhibitory Action of Quercetin on Eosinophil Activation In Vitro. Evid. -Based Complement. Altern. Med. 2013, 2013, 127105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, K.E.; Rice, C.M. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr. Top. Microbiol. Immunol. 2000, 242, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2013, 59, 318–327. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Hepatitis Report, 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Thomas, D.L.; Thio, C.L.; Martin, M.P.; Qi, Y.; Ge, D.; O’Huigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Hoofnagle, J.H. Course and outcome of hepatitis C. Hepatology 2002, 36, s21–s29. [Google Scholar] [CrossRef]
- Falck-Ytter, Y.; Kale, H.; Mullen, K.D.; Sarbah, S.A.; Sorescu, L.; McCullough, A.J. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann. Intern. Med. 2002, 136, 288–292. [Google Scholar] [CrossRef]
- Afdhal, N.; Reddy, K.R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and Sofosbuvir for Previously Treated HCV Genotype 1 Infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.-P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Park, H.; Saab, S.; Ahmed, A.; Dieterich, D.; Gordon, S.C. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment. Pharmacol. Ther. 2015, 41, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Burstow, N.J.; Mohamed, Z.; Gomaa, A.; Sonderup, M.W.; A Cook, N.; Waked, I.; Spearman, C.W.; Taylor-Robinson, S.D. Hepatitis C treatment: Where are we now? Int. J. Gen. Med. 2017, 10, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhatwal, J.; He, T.; Lopez-Olivo, M. Systematic Review of Modelling Approaches for the Cost Effectiveness of Hepatitis C Treatment with Direct-Acting Antivirals. Pharmacoeconomics 2016, 34, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.T.; Crespi, C.M.; Liu, N.M.; Vu, J.Q.; Ahmadieh, Y.; Wu, S.; Lin, S.; McClune, A.; Durazo, F.; Saab, S.; et al. A Phase I Dose Escalation Study Demonstrates Quercetin Safety and Explores Potential for Bioflavonoid Antivirals in Patients with Chronic Hepatitis C. Phytother. Res. 2015, 30, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, O.; Fontanes, V.; Raychaudhuri, S.; Loo, R.; Loo, J.; Arumugaswami, V.; Sun, R.; Dasgupta, A.; French, S.W. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology 2009, 50, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, D.; Ansari, I.H.; Mehle, A.; Striker, R. Fluorescence Resonance Energy Transfer-Based Intracellular Assay for the Conformation of Hepatitis C Virus Drug Target NS5A. J. Virol. 2012, 86, 8277–8286. [Google Scholar] [CrossRef] [Green Version]
- Lulu, S.S.; Thabitha, A.; Vino, S.; Priya, A.M.; Rout, M. Naringenin and quercetin—potential anti-HCV agents for NS2 protease targets. Nat. Prod. Res. 2015, 30, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Casaschi, A.; Wang, Q.; Richards, A.; Theriault, A. Intestinal apolipoprotein B secretion is inhibited by the flavonoid quercetin: Potential role of microsomal triglyceride transfer protein and diacylglycerol acyltransferase. Lipids 2002, 37, 647–652. [Google Scholar] [CrossRef]
- Gnoni, G.V.; Paglialonga, G.; Siculella, L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur. J. Clin. Investig. 2009, 39, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Harris, C.; Hernandez, C.; Carpentier, A.; Kaehlcke, K.; Rosenberg, A.R.; Ott, M. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med. 2010, 16, 1295–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prussia, A.; Thepchatri, P.; Snyder, J.P.; Plemper, R.K. Systematic Approaches towards the Development of Host-Directed Antiviral Therapeutics. Int. J. Mol. Sci. 2011, 12, 4027–4052. [Google Scholar] [CrossRef] [Green Version]
- Rojas, Á.; Del Campo, J.A.; Clement, S.; Lemasson, M.; García-Valdecasas, M.; Gil-Gómez, A.; Ranchal, I.; Bartosch, B.; Bautista, J.D.; Rosenberg, A.R.; et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci. Rep. 2016, 6, 31777. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell Mol. Life Sci. CMLS 2010, 67, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Jusoh, S.A. Molecular Docking and Molecular Dynamics Simulation Studies to Predict Flavonoid Binding on the Surface of DENV2 E Protein. Interdiscip. Sci. Comput. Life Sci. 2016, 9, 499–511. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. In Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009.
- Fried, J.R.; Gibbons, R.V.; Kalayanarooj, S.; Thomas, S.J.; Srikiatkhachorn, A.; Yoon, I.-K.; Jarman, R.G.; Green, S.; Rothman, A.L.; Cummings, D.A.T. Serotype-Specific Differences in the Risk of Dengue Hemorrhagic Fever: An Analysis of Data Collected in Bangkok, Thailand from 1994 to 2006. PLOS Negl. Trop. Dis. 2010, 4, e617. [Google Scholar] [CrossRef] [Green Version]
- Rajapakse, S.; Rodrigo, C.; Rajapakse, A. Treatment of dengue fever. Infect. Drug Resist. 2012, 5, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Low, J.G.H.; Ooi, E.E.; Vasudevan, S. Current Status of Dengue Therapeutics Research and Development. J. Infect. Dis. 2017, 215, S96–S102. [Google Scholar] [CrossRef] [Green Version]
- Whitehorn, J.; Yacoub, S.; Anders, K.L.; Macareo, L.R.; Cassetti, M.C.; Van, V.C.N.; Shi, P.-Y.; Wills, B.; Simmons, C.P. Dengue Therapeutics, Chemoprophylaxis, and Allied Tools: State of the Art and Future Directions. PLOS Negl. Trop. Dis. 2014, 8, e3025. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.T.U.; Mumtaz, A.; Naseem, R.; Ali, A.; Fatima, T.; Jabbar, T.; Ahmad, Z.; Ashfaq, U.A. Molecular Docking Based Screening of Plant Flavonoids as Dengue NS1 Inhibitors. Bioinformation 2014, 10, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.-F.; Zhang, L.; Chi, C.-W. Biological characteristics of dengue virus and potential targets for drug design. Acta Biochim. Biophys. Sin. 2008, 40, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilvel, P.; Lavanya, P.; Kumar, K.M.; Swetha, R.; Anitha, P.; Bag, S.; Sarveswari, S.; Vijayakumar, V.; Ramaiah, S.; Anbarasu, A. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents Dengue 2 viral assembly. Bioinformation 2013, 9, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Igbe, I.; Shen, X.-F.; Jiao, W.; Qiang, Z.; Deng, T.; Li, S.; Liu, W.-L.; Liu, H.-W.; Zhang, G.-L.; Wang, F. Dietary quercetin potentiates the antiproliferative effect of interferon-α in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase. Oncotarget 2017, 8, 113734–113748. [Google Scholar] [CrossRef] [PubMed]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.-Y.; García-Sastre, A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Jiménez, T.; Peña, L.M.-P.; Flores-Mendoza, L.; Sedeño-Monge, V.; Santos-López, G.; Rosas-Murrieta, N.; Reyes-Carmona, S.; Terán-Cabanillas, E.; Hernández, J.; Herrera-Camacho, I.; et al. Upregulation of the Suppressors of Cytokine Signaling 1 and 3 Is Associated with Arrest of Phosphorylated-STAT1 Nuclear Importation and Reduced Innate Response in Denguevirus-Infected Macrophages. Viral Immunol. 2016, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mendoza, L.K.; Estrada-Jiménez, T.; Sedeño-Monge, V.; Moreno, M.; Manjarrez, M.D.C.; González-Ochoa, G.; Peña, L.M.-P.; Reyes-Leyva, J. IL-10 and socs3 Are Predictive Biomarkers of Dengue Hemorrhagic Fever. Mediat. Inflamm. 2017, 2017, 5197592. [Google Scholar] [CrossRef] [Green Version]
- Palma-Ocampo, H.K.; Flores-Alonso, J.C.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Flores-Mendoza, L.; Herrera-Camacho, I.; Rosas-Murrieta, N.H.; Santos-López, G. Interferon lambda inhibits dengue virus replication in epithelial cells. Virol. J. 2015, 12, 150. [Google Scholar] [CrossRef] [Green Version]
- Ubol, S.; Phuklia, W.; Kalayanarooj, S.; Modhiran, N. Mechanisms of Immune Evasion Induced by a Complex of Dengue Virus and Preexisting Enhancing Antibodies. J. Infect. Dis. 2010, 201, 923–935. [Google Scholar] [CrossRef]
- Wiejak, J.; Dunlop, J.; Mackay, S.P.; Yarwood, S.J. Flavanoids induce expression of the suppressor of cytokine signalling 3 (SOCS3) gene and suppress IL-6-activated signal transducer and activator of transcription 3 (STAT3) activation in vascular endothelial cells. Biochem. J. 2013, 454, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasso-Miranda, C.; Herrera-Camacho, I.; Flores-Mendoza, L.K.; Dominguez, F.; Vallejo-Ruiz, V.; Sanchez-Burgos, G.G.; Pando-Robles, V.; Santos-Lopez, G.; Reyes-Leyva, J. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect. Drug Resist. 2019, 12, 1833–1852. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Rothman, A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 2006, 19, 429–436. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue Virus Pathogenesis: An Integrated View. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, K.I.; Jamil, B.; Rahim, M.; Islam, M.; Farhan, M.; Hasan, Z. Role of TNF α, IL-6 and CXCL10 in Dengue disease severity. Iran. J. Microbiol. 2018, 10, 202–207. [Google Scholar]
- Kuhn, J.H.; Becker, S.; Ebihara, H.; Geisbert, T.W.; Johnson, K.M.; Kawaoka, Y.; Lipkin, W.I.; Negredo, A.I.; Netesov, S.V.; Nichol, S.T.; et al. Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Arch. Virol. 2010, 155, 2083–2103. [Google Scholar] [CrossRef] [Green Version]
- Nanbo, A.; Watanabe, S.; Halfmann, P.; Kawaoka, Y. The spatio-temporal distribution dynamics of Ebola virus proteins and RNA in infected cells. Sci. Rep. 2013, 3, 1206. [Google Scholar] [CrossRef] [Green Version]
- Dapiaggi, F.; Pieraccini, S.; Potenza, D.; Vasile, F.; Podlipnik, Č. Designing Antiviral Substances Targeting the Ebola Virus Viral Protein 24. In Emerging and Reemerging Viral Pathogens; Academic Press: Cambridge, MA, USA, 2019; pp. 147–177. [Google Scholar] [CrossRef]
- Fanunza, E.; Frau, A.; Corona, A.; Tramontano, E. Antiviral Agents Against Ebola Virus Infection: Repositioning Old Drugs and Finding Novel Small Molecules. Annu. Rep. Med. Chem. 2018, 51, 135–173. [Google Scholar] [CrossRef]
- Baseler, L.; Chertow, D.S.; Johnson, K.M.; Feldmann, H.; Morens, D.M. The Pathogenesis of Ebola Virus Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 387–418. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Fais, A.; Pintus, F.; Santos-Buelga, C.; González-Paramás, A.M.; Piras, V.; Orrù, G.; Mameli, A.; Tramontano, E.; Frau, A. Broad-range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiol. 2017, 17, 159. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrbod, P.; Hudy, D.; Shyntum, D.; Markowski, J.; Łos, M.J.; Ghavami, S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Muthu, M.; Paul, D.; Ethiraj, G.; Chun, S.; Gopal, J. Nature nominee quercetin’s anti-influenza combat strategy-Demonstrations and remonstrations. Rev. Med. Virol. 2017, 27, e1930. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives—A biochemical perspective. Food Chem. 2020, 334, 127508. [Google Scholar] [CrossRef]
- Wilson, J.; von Itzstein, M. Recent Strategies in the Search for New Anti-Influenza Therapies. Curr. Drug Targets 2003, 4, 389–408. [Google Scholar] [CrossRef]
- Fan, X.; Hashem, A.; Chen, Z.; Li, C.; Doyle, T.; Zhang, Y.; Yi, Y.; Farnsworth, A.; Xu, K.; Li, Z.; et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol. 2015, 8, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, Y.S.; Kim, J.H.; Chung, H.S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019, 9, 12132. [Google Scholar] [CrossRef] [Green Version]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Rizky, W.C.; Jihwaprani, M.C.; Kindi, A.A.; Ansori, A.N.M.; Mushtaq, M. The pharmacological mechanism of quercetin as adjuvant therapy of COVID-19. Life Res. 2022, 5, 3. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rehman, T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. 2018, 27, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Mathew, S.; Suhail, M.; Ali, A.; Damanhouri, G.; Azhar, E.; Qadri, I. Docking studies of Pakistani HCV NS3 helicase: A possible antiviral drug target. PLoS One 2014, 9, e106339. [Google Scholar] [CrossRef] [Green Version]
- Chirumbolo, S. The Role of Quercetin, Flavonols and Flavones in Modulating Inflammatory Cell Function. Inflamm. Allergy-Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive Effect of Quercetin on Dendritic Cell Activation and Function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dodd, H.; Moser, E.; Sharma, R.; Braciale, T.J. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat. Immunol. 2011, 12, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.P.; Saiyed, Z.M.; Gandhi, N.H.; Ramchand, C.N. The Flavonoid, Quercetin, Inhibits HIV-1 Infection in Normal Peripheral Blood Mononuclear Cells. Am. J. Infect. Dis. 2009, 5, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Hodgson, K.; Miranda-Hernandez, S.; Hughes, S.; Kulur, A.B.; Ketheesan, N. Flavonoid quercetin–methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 2018, 26, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Souto, F.O.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Fattori, V.; Casagrande, R.; Fonseca, M.J.V.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, J.W.A. Quercetin Reduces Neutrophil Recruitment Induced by CXCL8, LTB4, and fMLP: Inhibition of Actin Polymerization. J. Nat. Prod. 2011, 74, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kasahara, Y.; Miyamoto, Y.; Takumi, O.; Kasai, T.; Onodera, K.; Kuwahara, M.; Oka, M.; Yoneda, Y.; Obika, S. Development of oligonucleotide-based antagonists of Ebola virus protein 24 inhibiting its interaction with karyopherin alpha 1. Org. Biomol. Chem. 2018, 16, 4456–4463. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Moonen, H.J.J.; Brauers, K.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Dietary Flavones and Flavonoles Are Inhibitors of Poly(ADP-ribose)polymerase-1 in Pulmonary Epithelial Cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; Mcanulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of Quercetin and EGCG on Mitochondrial Biogenesis and Immunity. Med. Sci. Sport. Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]








| Quercetin/Derivatives | Mechanism | References |
|---|---|---|
| Quercetin derivates | High binding activity on the capbinding site of influenza virus RNA polymerase PB2. | [133] |
| Quercetin | AntiHBV activity, inhibiting the formation of HBsAg and HBeAg. According to molecular docking, Quercetin forms very stable complexes with HBV | [135] |
| Quercetin | Latent HIV-1 gene expression is reactivated and nuclear factor κB nuclear translocation is induced | [39] |
| Quercetin | The docking studies show that Quercetin is a powerful inhibitor of the HCV NS2 protease. | [136] |
| Quercetin | Inhibits HSV entrance and NFB activation. | [47] |
| Quercetin | Inhibits 3CLpro and PLpro, with docking binding energies of 6.25 and 4.62 kcal/mol, respectively. | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shorobi, F.M.; Nisa, F.Y.; Saha, S.; Chowdhury, M.A.H.; Srisuphanunt, M.; Hossain, K.H.; Rahman, M.A. Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules 2023, 28, 938. https://doi.org/10.3390/molecules28030938
Shorobi FM, Nisa FY, Saha S, Chowdhury MAH, Srisuphanunt M, Hossain KH, Rahman MA. Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules. 2023; 28(3):938. https://doi.org/10.3390/molecules28030938
Chicago/Turabian StyleShorobi, Fauzia Mahanaz, Fatema Yasmin Nisa, Srabonti Saha, Muhammad Abid Hasan Chowdhury, Mayuna Srisuphanunt, Kazi Helal Hossain, and Md. Atiar Rahman. 2023. "Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions" Molecules 28, no. 3: 938. https://doi.org/10.3390/molecules28030938
APA StyleShorobi, F. M., Nisa, F. Y., Saha, S., Chowdhury, M. A. H., Srisuphanunt, M., Hossain, K. H., & Rahman, M. A. (2023). Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules, 28(3), 938. https://doi.org/10.3390/molecules28030938