A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology
Abstract
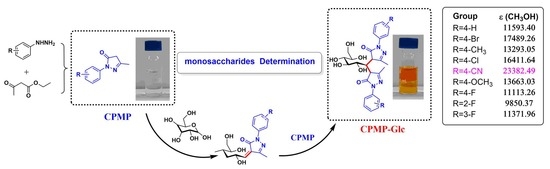
:1. Introduction
2. Results
2.1. UV-Vis Spectra of CPMP
2.2. Optimization of Derivatization Conditions by RSM
2.3. Mechanism Analysis of CPMP-Glc by UV-Vis and HRMS
2.4. Quality Control for P. cyrtonema Hua.
2.5. Method Validation
3. Materials and Methods
3.1. Reagent and Instrument
3.2. Synthesis of CPMP
3.3. Investigation of Spectroscopic Characteristics
3.4. Optimizing Derivatization Condition by RSM [16]
3.5. High-Performance Liquid Chromatography
3.6. Preparation of P. cyrtonema Hua. Polysaccharides (PCPs)
3.7. Monosaccharide Determination
4. Discussion
4.1. Synthesis of CPMP
4.2. Establishment of the HPLC Method
4.3. Mechanism Analysis
4.4. Comparative Analysis with Existing Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Li, H.; Sheng, Y.; Li, W.; Yuan, L. Recent Advances in Molecular Fluorescent Probes for CYP450 Sensing and Imaging. Chemosensors 2022, 10, 304. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and biosensing applications of spiropyran and its derivatives—A review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Ji, X.-L.; Guo, J.-H.; Pan, F.-B.; Kuang, F.-J.; Chen, H.-M.; Guo, X.-D.; Liu, Y.-Q. Structural elucidation and antioxidant activities of a neutral polysaccharide from Arecanut (Areca catechu L.). Front. Nutr. 2022, 9, 853115. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Ding, D.; Gao, J.; Hao, L.; Guo, X.; Liu, Y. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Pauk, V.; Pluhacek, T.; Havlicek, V.; Lemr, K. Ultra-high performance supercritical fluid chromatography-mass spectrometry procedure for analysis of monosaccharides from plant gum binders. Anal. Chim. Acta 2017, 989, 112–120. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2020 ed.; Part III; China Medical Science and Technology Press: Beijing, China, 2020; p. 566. [Google Scholar]
- Sharma, U.; Bhandari, P.; Kumar, N.; Singh, B. Simultaneous Determination of Ten Sugars in Tinospora cordifolia by Ultrasonic Assisted Extraction and LC-ELSD. Chromatographia 2010, 71, 633–638. [Google Scholar] [CrossRef]
- Long, L.-L.; Han, Y.-Y.; Liu, W.-G.; Chen, Q.; Yin, D.-D.; Li, L.-L.; Yuan, F.; Han, Z.-X.; Gong, A.-H.; Wang, K. Simultaneous Discrimination of Hypochlorite and Single Oxygen during Sepsis by a Dual-Functional Fluorescent Probe. Anal. Chem. 2020, 92, 6072–6080. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, D.; Wang, T.; Xiao, J.; Yu, L. A Label-Free Colorimetric Assay Based on Gold Nanoparticles for the Detection of H2O2 and Glucose. Chemosensors 2022, 10, 100. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Ren, T.-B.; Yang, F.-Y.; Yuan, L. Rational design of far red to near-infrared rhodamine analogues with huge Stokes shifts for single-laser excitation multicolor imaging. Chin. Chem. Lett. 2021, 32, 3890–3894. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.-W.; Zhu, S.; Yin, H.-P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Luo, L.; Cui, Z.; Wang, N.; Shu, Y.-M.; Wang, K.-P. Pharmacokinetics, biodistribution and receptor mediated endocytosis of a natural Angelica sinensis polysaccharide. Artif. Cells Nanomed. Biotechnol. 2018, 46, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Li, X.; Wang, Y.; Zhang, X.; Jia, H.; Guo, L.; Huang, L.; Gao, W. Comparative studies on characterization, saccharide mapping and antiglycation activity of polysaccharides from different Polygonatum ssp. J. Pharm. Biomed. Anal. 2020, 186, 113243. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.-X.; Zhao, Y.-Y.; Du, J.; Wang, B. Synthesis of naphthalimide-type chemsensor and its application in quality evaluation for polygonatum sibiricum Red. Front. Chem. 2022, 10, 969014. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Liu, H.; Li, X.; Wang, B. tert-Butyl nitrite triggered radical cascade reaction for synthesizing isoxazoles by a one-pot multicomponent strategy. Chem. Commun. 2022, 58, 9152–9155. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.L.; Tong, G.Y.; Li, Y.; Lei, M.N.; Wu, H.; Wang, B.; Hu, R.F. Development of a novel UHPLC-UV combined with UHPLC-QTOF/MS fingerprint method for the comprehensive evaluation of Nao-Luo-Xin-Tong: Multi-wavelength setting based on traditional Chinese medicinal prescription composition. Anal. Methods 2019, 11, 6092–6102. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Cao, Y.; Zhang, T.; Guo, H.; Wang, B. A novel method for investigating the mechanism of the anti-rheumatoid arthritis activity of Angelicae pubescentis radix by integrating UHPLC–QTOF/MS and network pharmacology. Biomed. Chromatogr. 2022, 36, e5389. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.-Y.; Zhu, G.-Q. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Huang, G.; He, J.-X.; Zhang, X.; Feng, M.-M.; Tan, Y.; Lv, C.-C.; Huang, H.; Jin, Z. Applications of Lambert-Beer law in the preparation and performance evaluation of graphene modified asphalt. Constr. Build. Mater. 2020, 273, 121582. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Chen, F.; Wang, Y.-Q.; Wang, L.-N.; Fu, H.-Y.; Zheng, F.-P.; Beecher, L. Optimization of reactions between reducing sugars and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology. Food Chem. 2018, 254, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Al-Karagoly, H.; Allafchian, A.R.; Jalali, S.A.H.; Al-Kelabi, T.; Muhannad, M. Production and characterization of biocompatible nanofibrous scaffolds made of β-sitosterol loaded polyvinyl alcohol/tragacanth gum composites. Nanotechnology 2022, 33, 085102. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Zhu, X.; Zhang, H. Glucose detection via glucose-induced disaggregation of ammonium-modified tetraphenylethylene from polyanion. Sens. Actuators B Chem. 2017, 246, 819–825. [Google Scholar] [CrossRef]
- Hao, T.; Wei, X.; Nie, Y.; Xu, Y.; Lu, K.; Yan, Y.; Zhou, Z. Surface modification and ratiometric fluorescence dual function enhancement for visual and fluorescent detection of glucose based on dual-emission quantum dots hybrid. Sens. Actuators B Chem. 2016, 230, 70–76. [Google Scholar] [CrossRef]
- Qu, Z.B.; Zhou, X.; Gu, L.; Lan, R.; Sun, D.; Yu, D.; Shi, G. Boronic acid functionalized graphene quantum dots as a fluorescent probe for selective and sensitive glucose determination in microdialysate. Chem. Commun. 2013, 49, 9830–9832. [Google Scholar] [CrossRef]
- Yan, Y.-Z.; Li, S.-Q.; Zhang, Z.-H.; Qu, J.-B.; Wang, J.-Y. A sensitive bio-probe for tracking lipid droplets with large Stokes shift and its application in cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119988. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Chen, H.-L.; Luo, L.; Zhou, Z.-P.; Wang, Y.-X.; Gao, T.-Y.; Yang, L.; Peng, T.; Wu, M.-Y. Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria. Carbohydr. Polym. 2021, 267, 118219. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhou, R.; Cheng, F.; Tang, X.; Lao, J.; Xu, L.; He, W.; Wan, D.; Zeng, H.; et al. Polygonatum cyrtonema Hua Polysaccharides Protect BV2 Microglia Relief Oxidative Stress and Ferroptosis by Regulating NRF2/HO-1 Pathway. Molecules 2022, 27, 7088. [Google Scholar] [CrossRef]
- Chiu, W.-J.; Chen, H.-R.; Barve, I.J.; Sun, C.-M. Rh(III)-Catalyzed (4 + 1) Annulation of Pyrazol-3-ones with Alkynoates via Ortho-Alkenylation/Cyclization Cascade: Synthesis of Indazole-Fused Pyrazoles. J. Org. Chem. 2022, 87, 12109–12114. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.-F.; Hu, S.-L.; Li, C.-P.; Wang, Q.; Wang, R.; Han, X.; Zhou, Y.; Liu, H. Catalytic System-Controlled Divergent Reaction Strategies for the Construction of Diversified Spiropyrazolone Skeletons from Pyrazolidinones and Diazopyrazolones. Angew. Chem. Int. Ed. 2021, 60, 21327–21333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.-P.; Li, T.-J.; Liu, G.-Y.; Wang, H.-M.; Lu, K. p-Toluenesulphonic acid-promoted, I2-catalysed sulphenylation of pyrazolones with aryl sulphonyl hydrazides. Chem. Commun. 2014, 50, 13121–13123. [Google Scholar] [CrossRef] [PubMed]






| Different Groups | ε (L/mol/cm) CH3OH | ε (L/mol/cm) CH3CH2OH | ε (L/mol/cm) CH3CN |
|---|---|---|---|
| 4-H | 11,593.40 | 14,219.19 | 13,611.52 |
| 4-Br | 17,489.26 | 16,003.25 | 14,516.14 |
| 4-CH3 | 13,293.05 | 13,634.17 | 13,941.38 |
| 4-Cl | 16,411.64 | 17,587.45 | 14,254.44 |
| 4-CN | 23,382.49 | 20,852.47 | 19,712.28 |
| 4-OCH3 | 13,663.03 | 15,953.65 | 16,379.34 |
| 4-F | 11,113.26 | 13,659.38 | 13,376.55 |
| 3-F | 11,371.96 | 16,803.28 | 16,541.72 |
| 2-F | 9850.37 | 10,301.37 | 10,620.38 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 100.36 | 14 | 7.17 | 9.24 | <0.0001 |
| A | 2.61 | 1 | 2.61 | 3.37 | 0.0878 |
| B | 23.24 | 1 | 23.24 | 29.95 | <0.0001 |
| C | 0.1008 | 1 | 0.1008 | 0.1300 | 0.7239 |
| D | 47.20 | 1 | 47.20 | 60.84 | <0.0001 |
| AB | 0.0225 | 1 | 0.0225 | 0.0290 | 0.8672 |
| AC | 0.2025 | 1 | 0.2025 | 0.2610 | 0.6174 |
| AD | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 |
| BC | 0.2025 | 1 | 0.2025 | 0.2610 | 0.6174 |
| BD | 0.5625 | 1 | 0.5625 | 0.7250 | 0.4088 |
| CD | 0.4225 | 1 | 0.4225 | 0.5446 | 0.4727 |
| A2 | 13.44 | 1 | 13.44 | 17.32 | 0.0010 |
| B2 | 11.42 | 1 | 11.42 | 14.72 | 0.0018 |
| C2 | 10.17 | 1 | 10.17 | 13.10 | 0.0028 |
| D2 | 3.79 | 1 | 3.79 | 4.89 | 0.0442 |
| Residual | 10.86 | 14 | 0.7759 | ||
| Lack of Fit | 3.04 | 10 | 0.3039 | 0.1554 | 0.9921 |
| Pure Error | 7.82 | 4 | 1.96 | ||
| Cor Total | 111.23 | 28 | |||
| R2 = 0.9023 | R2Adj = 0.8047 | R2Pred = 0.7327 | CV = 1.27% | Adeq Precision = 10.6553 | |
| Monosaccharide | Concentration (μg/mg) | Ratio |
|---|---|---|
| Gal | 1.88 | 0.58 |
| Glc | 5.62 | 1.73 |
| Man | 3.25 | 1 |
| Monosaccharides | tR (min) | Calibration Curves | r2 | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| Gal | 4.78 | y = 25116x + 12.52 | 0.9992 | 1-21 | 2.12 × 10−3 | 6.42 × 10−3 |
| Glc | 5.92 | y = 10871x+16.178 | 0.9995 | 5-104 | 5.22 × 10−3 | 15.81 × 10−3 |
| Man | 12.03 | y = 14698x + 6.1496 | 0.9997 | 3-52 | 5.66 × 10−3 | 17.15 × 10−3 |
| Monosaccharides | Content in Sample (μg/mL) | Precision | Stability | Repeatability | Sample Adding Recovery |
|---|---|---|---|---|---|
| RSD (%) | RSD (%) | RSD (%) | (%) | ||
| Gal | 20 | 0.78 | 0.72 | 0.66 | 103.37 |
| Glc | 20 | 0.44 | 0.31 | 1.09 | 101.25 |
| Man | 20 | 0.98 | 1.02 | 1.15 | 99.87 |
| Variable | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Temperature (A, °C) | 50 | 70 | 90 |
| Time (B, min) | 30 | 60 | 90 |
| Alkali concentration (C, mol/L) | 0.3 | 0.4 | 0.5 |
| CPMP concentration (D, mol/L) | 0.4 | 0.6 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhao, Y.; Chen, L.; Du, J.; Guo, H.; Wang, B. A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology. Molecules 2023, 28, 2186. https://doi.org/10.3390/molecules28052186
Liu H, Zhao Y, Chen L, Du J, Guo H, Wang B. A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology. Molecules. 2023; 28(5):2186. https://doi.org/10.3390/molecules28052186
Chicago/Turabian StyleLiu, Hui, Yuanyuan Zhao, Leijing Chen, Jiao Du, Hongyan Guo, and Bin Wang. 2023. "A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology" Molecules 28, no. 5: 2186. https://doi.org/10.3390/molecules28052186
APA StyleLiu, H., Zhao, Y., Chen, L., Du, J., Guo, H., & Wang, B. (2023). A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology. Molecules, 28(5), 2186. https://doi.org/10.3390/molecules28052186