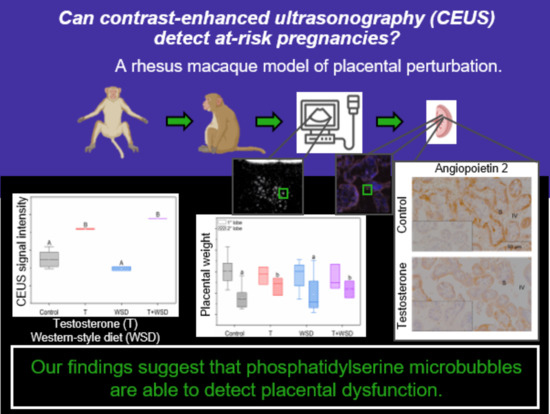
Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques
Abstract
:1. Introduction
2. Results
2.1. In Vivo Imaging with MB-PS
2.2. Fetal and Placental Weights
2.3. Immunofluorescence for MB-PS and Immunohistochemistry for Inflammation Markers
3. Discussion
4. Materials and Methods
4.1. Animal Ethics and Care
4.2. Animal Model
4.3. MB-PS Synthesis
4.4. In Vivo Imaging and Tissue Collection
4.5. Immunofluorescence for MB-PS and Immunohistochemistry for Inflammation Markers
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The Unique Microbiome and Innate Immunity During Pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Orsaria, M.; Liviero, S.; Rossetti, E.; Pittini, C.; Driul, L.; Londero, A.P.; Mariuzzi, L. Placental Acute Inflammation Infiltrates and Pregnancy Outcomes: A Retrospective Cohort Study. Sci. Rep. 2021, 11, 24165. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Lokki, A.I.; Fleming, S.D.; Regal, J.F. Editorial: Innate Immunity in Normal and Adverse Pregnancy. Front. Immunol. 2021, 12, 646596. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Bulla, R.; Salmon, J.E.; Tedesco, F. The Complement System in the Pathophysiology of Pregnancy. Mol. Immunol. 2006, 43, 68–77. [Google Scholar] [CrossRef]
- Lim, R.; Lappas, M. Decreased Expression of Complement 3a Receptor (C3aR) in Human Placentas from Severe Preeclamptic Pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 194–198. [Google Scholar] [CrossRef]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Francis, E.C.; Dabelea, D.; Boyle, K.E.; Jansson, T.; Perng, W. Maternal Diet Quality Is Associated with Placental Proteins in the Placental Insulin/Growth Factor, Environmental Stress, Inflammation, and MTOR Signaling Pathways: The Healthy Start ECHO Cohort. J. Nutr. 2022, 152, 816–825. [Google Scholar] [CrossRef]
- Jaworsky, K.; Ebersole, J.L.; Planinic, P.; Basu, A. Associations of Diet with Cardiometabolic and Inflammatory Profiles in Pregnant Women at Risk for Metabolic Complications. Int. J. Environ. Res. Public Health 2021, 18, 11105. [Google Scholar] [CrossRef]
- Ma, J.-S.; Mei, X.; Niu, Y.-X.; Li, Q.-G.; Jiang, X.-F. Risk Factors and Adverse Pregnancy Outcomes of Succenturiate Placenta: A Case-Control Study. J. Reprod. Med. 2016, 61, 139–144. [Google Scholar]
- Roberts, V.H.J.; Castro, J.N.; Conrad, D.F.; Lewis, A.D.; Lo, J.O. Contemporary Rhesus Macaque Fetal and Placental Growth Demographics: A Resource for Laboratory Animal Researchers. Am. J. Primatol. 2023; under revision. [Google Scholar]
- Kitchen, F.L.; Jack, B.W. Prenatal Screening. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jacquier, M.; Arthuis, C.; Grévent, D.; Bussières, L.; Henry, C.; Millischer-Bellaiche, A.-E.; Mahallati, H.; Ville, Y.; Siauve, N.; Salomon, L.J. Dynamic Contrast Enhanced Magnetic Resonance Imaging: A Review of Its Application in the Assessment of Placental Function. Placenta 2021, 114, 90–99. [Google Scholar] [CrossRef]
- Mai, Z.-S.; Chen, Y.-Q.; Liao, M.; Ma, C.; Han, Y.-B. Early Detection of a Cesarean Scar Pregnancy with Placenta Increta by Contrast-Enhanced Ultrasound in the First Trimester: A Case Report and Literature Review. J. Obstet. Gynaecol. Res. 2022, 48, 251–255. [Google Scholar] [CrossRef]
- Roberts, V.H.; Frias, A.E. Contrast-Enhanced Ultrasound for the Assessment of Placental Development and Function. Biotechniques 2020, 69, 392–399. [Google Scholar] [CrossRef]
- Silva, P.; Maronezi, M.C.; Padilha-Nakaghi, L.C.; Gasser, B.; Pavan, L.; Nogueira Aires, L.P.; Russo, M.; Spada, S.; Ramirez Uscategui, R.A.; Moraes, P.C.; et al. Contrast-Enhanced Ultrasound Evaluation of Placental Perfusion in Brachicephalic Bitches. Theriogenology 2021, 173, 230–240. [Google Scholar] [CrossRef]
- Oh, K.Y.; Roberts, V.H.J.; Schabel, M.C.; Grove, K.L.; Woods, M.; Frias, A.E. Gadolinium Chelate Contrast Material in Pregnancy: Fetal Biodistribution in the Nonhuman Primate. Radiology 2015, 276, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Prola-Netto, J.; Woods, M.; Roberts, V.H.J.; Sullivan, E.L.; Miller, C.A.; Frias, A.E.; Oh, K.Y. Gadolinium Chelate Safety in Pregnancy: Barely Detectable Gadolinium Levels in the Juvenile Nonhuman Primate after in Utero Exposure. Radiology 2018, 286, 122–128. [Google Scholar] [CrossRef]
- Kaufmann, B.A.; Wei, K.; Lindner, J.R. Contrast Echocardiography. Curr. Probl. Cardiol. 2007, 32, 51–96. [Google Scholar] [CrossRef]
- Lindner, J.R. Contrast Echocardiography: Current Status and Future Directions. Heart Br. Card. Soc. 2021, 107, 18–24. [Google Scholar] [CrossRef]
- Roberts, V.H.; Lo, J.O.; Salati, J.A.; Lewandowski, K.S.; Lindner, J.R.; Morgan, T.K.; Frias, A.E. Quantitative Assessment of Placental Perfusion by Contrast-Enhanced Ultrasound in Macaques and Human Subjects. Am. J. Obstet. Gynecol. 2016, 214, 369.e1–369.e8. [Google Scholar] [CrossRef] [Green Version]
- Salati, J.A.; Roberts, V.H.J.; Schabel, M.C.; Lo, J.O.; Kroenke, C.D.; Lewandowski, K.S.; Lindner, J.R.; Grove, K.L.; Frias, A.E. Maternal High Fat Diet Reversal Improves Placental Hemodynamics in a Nonhuman Primate Model of Diet-Induced Obesity. Int. J. Obes. 2019, 43, 906–916. [Google Scholar] [CrossRef]
- Maruyama, H.; Matsutani, S.; Saisho, H.; Mine, Y.; Kamiyama, N.; Hirata, T.; Sasamata, M. Real-Time Blood-Pool Images of Contrast Enhanced Ultrasound with Definity in the Detection of Tumour Nodules in the Liver. Br. J. Radiol. 2005, 78, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Matsutani, S.; Saisho, H.; Mine, Y.; Yuki, H.; Miyata, K. Different Behaviors of Microbubbles in the Liver: Time-Related Quantitative Analysis of Two Ultrasound Contrast Agents, Levovist and Definity. Ultrasound Med. Biol. 2004, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pang, E.H.T.; Chan, A.; Ho, S.G.; Harris, A.C. Contrast-Enhanced Ultrasound of the Liver: Optimizing Technique and Clinical Applications. AJR Am. J. Roentgenol. 2018, 210, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Song, J.; Xu, F.; Klibanov, A.L.; Singbartl, K.; Ley, K.; Kaul, S. Noninvasive Ultrasound Imaging of Inflammation Using Microbubbles Targeted to Activated Leukocytes. Circulation 2000, 102, 2745–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, B.; Packwood, W.; Xie, A.; Belcik, J.T.; Taylor, R.P.; Zhao, Y.; Davidson, B.P.; Lindner, J.R. Echocardiographic Ischemic Memory Imaging Through Complement-Mediated Vascular Adhesion of Phosphatidylserine-Containing Microbubbles. JACC Cardiovasc. Imaging 2016, 9, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Derieppe, M.; Lammertink, B.; Bos, C.; Moonen, C. Microbubble-Assisted Ultrasound-Induced Transient Phosphatidylserine Translocation. Ultrasound Med. Biol. 2017, 43, 838–851. [Google Scholar] [CrossRef]
- Davidson, B.P.; Hodovan, J.; Layoun, M.E.; Golwala, H.; Zahr, F.; Lindner, J.R. Echocardiographic Ischemic Memory Molecular Imaging for Point-of-Care Detection of Myocardial Ischemia. J. Am. Coll. Cardiol. 2021, 78, 1990–2000. [Google Scholar] [CrossRef]
- Lindner, J.R.; Song, J.; Christiansen, J.; Klibanov, A.L.; Xu, F.; Ley, K. Ultrasound Assessment of Inflammation and Renal Tissue Injury with Microbubbles Targeted to P-Selectin. Circulation 2001, 104, 2107–2112. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.R. Detection of Myocarditis with Molecular Echo Imaging: Another Potential Application for the Phosphatidyl Serine Microbubble. Circ. Cardiovasc. Imaging 2016, 9, e005249. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.R. The Potential for Retained Microbubbles: To Imaging… and beyond. J. Am. Coll. Cardiol. 2021, 78, 2001–2003. [Google Scholar] [CrossRef]
- Uszyński, M.; Uszyński, W.; Zekanowska, E. P-Selectin in Placenta and Gestational Myometrium: Its Measurements and Hypothetical Role in Hemostasis of Placental Bed after Labor. J. Perinat. Med. 2008, 36, 213–216. [Google Scholar] [CrossRef]
- Charnock-Jones, D.S. Soluble Flt-1 and the Angiopoietins in the Development and Regulation of Placental Vasculature. J. Anat. 2002, 200, 607–615. [Google Scholar] [CrossRef]
- Keenan-Devlin, L.S.; Caplan, M.; Freedman, A.; Kuchta, K.; Grobman, W.; Buss, C.; Adam, E.K.; Entringer, S.; Miller, G.E.; Borders, A.E.B. Using Principal Component Analysis to Examine Associations of Early Pregnancy Inflammatory Biomarker Profiles and Adverse Birth Outcomes. Am. J. Reprod. Immunol. 2021, 86, e13497. [Google Scholar] [CrossRef]
- Prairie, E.; Côté, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The Determinant Role of IL-6 in the Establishment of Inflammation Leading to Spontaneous Preterm Birth. Cytokine Growth Factor Rev. 2021, 59, 118–130. [Google Scholar] [CrossRef]
- Sacks, G.P.; Studena, K.; Sargent, K.; Redman, C.W. Normal Pregnancy and Preeclampsia Both Produce Inflammatory Changes in Peripheral Blood Leukocytes Akin to Those of Sepsis. Am. J. Obstet. Gynecol. 1998, 179, 80–86. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Fest, S.; Sehmsdorf, U.S.; Hagen, E.; Klapp, B.F.; Arck, P.C. Upregulation of Decidual P-Selectin Expression Is Associated with an Increased Number of Th1 Cell Populations in Patients Suffering from Spontaneous Abortions. Cell. Immunol. 2001, 213, 94–103. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Morgan, T.K.; Bednarek, P.; Morita, M.; Burton, G.J.; Lo, J.O.; Frias, A.E. Early First Trimester Uteroplacental Flow and the Progressive Disintegration of Spiral Artery Plugs: New Insights from Contrast-Enhanced Ultrasound and Tissue Histopathology. Hum. Reprod. Oxf. Engl. 2017, 32, 2382–2393. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.; Roberts, V.H.J.; Gaffney, J.; Takahashi, D.L.; Morgan, T.; Lo, J.O.; Stouffer, R.L.; Frias, A.E. Maternal High-Fat Diet Consumption and Chronic Hyperandrogenemia Are Associated with Placental Dysfunction in Female Rhesus Macaques. Endocrinology 2019, 160, 1937–1949. [Google Scholar] [CrossRef]
- Atkinson, T.; Lindner, J.R. Ultrasound Molecular Imaging of Endothelial Cell Activation and Damage in Atherosclerosis. In Cardiovascular Imaging: Arterial and Aortic Valve Inflammation and Calcification; Aikawa, E., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 39–63. ISBN 978-3-319-09268-3. [Google Scholar]
- Brown, E.; Lindner, J.R. Ultrasound Molecular Imaging: Principles and Applications in Cardiovascular Medicine. Curr. Cardiol. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Kosareva, A.; Abou-Elkacem, L.; Chowdhury, S.; Lindner, J.R.; Kaufmann, B.A. Seeing the Invisible-Ultrasound Molecular Imaging. Ultrasound Med. Biol. 2020, 46, 479–497. [Google Scholar] [CrossRef] [Green Version]
- Strachinaru, M.; Cate, F.J. ten Microbubble Enhanced Echocardiography in Current Cardiology Practice. Rev. Cardiovasc. Med. 2022, 23, 202. [Google Scholar] [CrossRef]
- Bianchi, C.; Taricco, E.; Cardellicchio, M.; Mandò, C.; Massari, M.; Savasi, V.; Cetin, I. The Role of Obesity and Gestational Diabetes on Placental Size and Fetal Oxygenation. Placenta 2021, 103, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Sferruzzi-Perri, A.N.; Coan, P.M.; Constancia, M.; Burton, G.J. Placental Efficiency and Adaptation: Endocrine Regulation. J. Physiol. 2009, 587, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- Nteeba, J.; Varberg, K.M.; Scott, R.L.; Simon, M.E.; Iqbal, K.; Soares, M.J. Poorly Controlled Diabetes Mellitus Alters Placental Structure, Efficiency, and Plasticity. BMJ Open Diabetes Res. Care 2020, 8, e001243. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Redmer, D.A. Angiogenesis in the Placenta. Biol. Reprod. 2001, 64, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2020, 11, 604000. [Google Scholar] [CrossRef]
- Posma, E.; Moes, H.; Heineman, M.J.; Faas, M.M. The Effect of Testosterone on Cytokine Production in the Specific and Non-Specific Immune Response. Am. J. Reprod. Immunol. 2004, 52, 237–243. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Guo, X.; Jia, W.; Liu, G.; Zhang, J.; Li, J.; Cui, P.; Sferruzzi-Perri, A.N.; Han, Y.; et al. Hyperandrogenism and Insulin Resistance Induce Gravid Uterine Defects in Association with Mitochondrial Dysfunction and Aberrant Reactive Oxygen Species Production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E794–E809. [Google Scholar] [CrossRef]
- Resko, J.A.; Buhl, A.E.; Phoenix, C.H. Treatment of Pregnant Rhesus Macaques with Testosterone Propionate: Observations on Its Fate in the Fetus. Biol. Reprod. 1987, 37, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- Bishop, C.V.; Luo, F.; Gao, L.; Fei, S.S.; Slayden, O.D. Mild Hyperandrogenemia in Presence/Absence of a High-Fat, Western-Style Diet Alters Secretory Phase Endometrial Transcriptome in Nonhuman Primates. F&S Sci. 2020, 1, 172–182. [Google Scholar] [CrossRef]
- Bishop, C.V.; Mishler, E.C.; Takahashi, D.L.; Reiter, T.E.; Bond, K.R.; True, C.A.; Slayden, O.D.; Stouffer, R.L. Chronic Hyperandrogenemia in the Presence and Absence of a Western-Style Diet Impairs Ovarian and Uterine Structure/Function in Young Adult Rhesus Monkeys. Hum. Reprod. Oxf. Engl. 2018, 33, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.V.; Stouffer, R.L.; Takahashi, D.L.; Mishler, E.C.; Wilcox, M.C.; Slayden, O.D.; True, C.A. Chronic Hyperandrogenemia and Western-Style Diet Beginning at Puberty Reduces Fertility and Increases Metabolic Dysfunction during Pregnancy in Young Adult, Female Macaques. Hum. Reprod. Oxf. Engl. 2018, 33, 694–705. [Google Scholar] [CrossRef]
- True, C.A.; Takahashi, D.L.; Burns, S.E.; Mishler, E.C.; Bond, K.R.; Wilcox, M.C.; Calhoun, A.R.; Bader, L.A.; Dean, T.A.; Ryan, N.D.; et al. Chronic Combined Hyperandrogenemia and Western-Style Diet in Young Female Rhesus Macaques Causes Greater Metabolic Impairments Compared to Either Treatment Alone. Hum. Reprod. Oxf. Engl. 2017, 32, 1880–1891. [Google Scholar] [CrossRef] [Green Version]
- Varlamov, O.; Bishop, C.V.; Handu, M.; Takahashi, D.; Srinivasan, S.; White, A.; Roberts, C.T. Combined Androgen Excess and Western-Style Diet Accelerates Adipose Tissue Dysfunction in Young Adult, Female Nonhuman Primates. Hum. Reprod. Oxf. Engl. 2017, 32, 1892–1902. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, C.M.; Bhattacharya, S.; Repping, S.; Mastenbroek, S.; Kamath, M.S.; Marjoribanks, J.; Boivin, J. Female Subfertility. Nat. Rev. Dis. Primer 2019, 5, 7. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.C.; Lo, J.O.; Romero Jimenez, G.; Lindner, J.R.; Slayden, O.D.; Roberts, V.H.J. Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques. Molecules 2023, 28, 2894. https://doi.org/10.3390/molecules28072894
Wilson RC, Lo JO, Romero Jimenez G, Lindner JR, Slayden OD, Roberts VHJ. Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques. Molecules. 2023; 28(7):2894. https://doi.org/10.3390/molecules28072894
Chicago/Turabian StyleWilson, Rachel C., Jamie O. Lo, Gabriel Romero Jimenez, Jonathan R. Lindner, Ov D. Slayden, and Victoria H. J. Roberts. 2023. "Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques" Molecules 28, no. 7: 2894. https://doi.org/10.3390/molecules28072894
APA StyleWilson, R. C., Lo, J. O., Romero Jimenez, G., Lindner, J. R., Slayden, O. D., & Roberts, V. H. J. (2023). Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques. Molecules, 28(7), 2894. https://doi.org/10.3390/molecules28072894