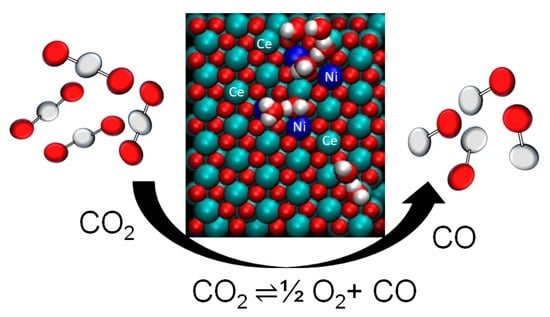
Nanoshaped Cerium Oxide with Nickel as a Non-Noble Metal Catalyst for CO2 Thermochemical Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoshaped Ceria
2.2. Metal-Decorated Ceria
3. Preparation and Methods
3.1. Preparation of Catalysts
3.2. Materials Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perathoner, S.; Centi, G. CO2 Recycling: A key strategy to introduce green energy in the chemical production chain. ChemSusChem 2014, 7, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Bogeat, A.; Blanco, G.; Pérez-Sagasti, J.J.; Escudero, C.; Pellegrin, E.; Herrera, F.C.; Pintado, J.M. Thermocatalytic CO2 Conversion over a Nickel-Loaded Ceria Nanostructured Catalyst: ANAP-XPS Study. Materials 2021, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.A. Angelini, The changing paradigm in CO2utilization. J. CO2 Util. 2013, 3–4, 65–73. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Farooqui, A.; Bose, A.; Ferrero, D.; Llorca, J.; Santarelli, M. Simulation of two-step redox recycling of non-stoichiometric ceria with thermochemical dissociation of CO2/H2O in moving bed reactors -Part II: Techno-economic analysis and integration with 100 MW oxyfuel power plant with carbon capture. Chem. Eng. Sci. 2019, 226, 114873. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. A review on solar thermal syngas production via redox pair-based water/carbon dioxide splitting thermochemical cycles. Renew. Sustain. Energy Rev. 2015, 42, 254–285. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Call, F.; Simon, H.; Sattler, C.; Schmcker, M.; Pitz-Paal, R. Materials-related aspects of thermochemical water and carbon dioxide splitting: A review. Materials 2012, 5, 2015–2054. [Google Scholar] [CrossRef] [Green Version]
- Pappacena, A.; Boaro, M.; Armelao, L.; Llorca, J.; Trovarelli, A. Water splitting reaction on Ce0.15Zr0.85O2 driven by surface heterogeneity. Catal. Sci. Technol. 2016, 6, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.E.; Pica, A.M.; Marocco, P.; Ferrero, D.; Lanzini, A.; Fiorilli, S.; Llorca, J.; Santarelli, M. Assessment of kinetic model for ceria oxidation for chemical-looping CO2 dissociation. Chem. Eng. J. 2018, 346, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, C.; Lord, M.S.; Lovell, E.; Wong, R.J.; Jung, M.S.; Oscar, D.; Mann, R.; Amal, R. Oxygen-Vacancy Engineering of Cerium-Oxide Nanoparticles for Antioxidant Activity. ACS Omega 2019, 4, 9473–9479. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef]
- Sun, X.; Gong, C.; Lv, G.; Bin, F.; Song, C. Effect of Ce/Zr molar ratio on the performance of Cu–Cex–Zr1−x/TiO2 catalyst for selective catalytic reduction of NOx with NH3 in diesel exhaust. Mater. Res. Bull. 2014, 60, 341–347. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Rangel, R.; Bartolo-Pérez, P.; Martínez, E.; Trejo-Cruz, X.A.; Díaz, G.; Galván, D.H. Catalytic activity and X-ray photoelectron spectroscopy performance of Bi2MoxW(1−x)O6 solid-solutions. Catal. Sci. Technol. 2012, 2, 847–852. [Google Scholar] [CrossRef]
- Jung, J.C.; Lee, H.; Kim, H.; Chung, Y.M.; Kim, T.J.; Lee, S.J.; Oh, S.H.; Kim, Y.S.; Song, I.K. Effect of Oxygen Capacity and Oxygen Mobility of Pure Bismuth Molybdate and Multicomponent Bismuth Molybdate on their Catalytic Performance in the Oxidative Dehydrogenation of n-Butene to 1,3-Butadiene. Catal. Lett. 2008, 124, 262–267. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. A thermochemical study of ceria: Exploiting an old material for new modes of energy conversion and CO2 mitigation. Philos. Trans. R. Soc. A 2010, 368, 3269–3294. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, R.R.; Takalkar, G.; Sutar, P.; Kumar, A.; AlMomani, F.; Khraisheh, M. A decade of ceria based solar thermochemical H2O/CO2 splitting cycle. Int. J. Hydrogen Energy 2019, 44, 34–60. [Google Scholar] [CrossRef]
- Ioannou, M.E.; Pouroutzidou, G.K.; Chatzimentor, I.; Tsamesidis, I.; Florini, N.; Tsiaoussis, I.; Lymperaki, E.; Komninou, P.; Kontonasaki, E. Synthesis and Characterization of Cerium Oxide Nanoparticles: Effect of Cerium Precursor to Gelatin Ratio. Appl. Sci. 2023, 13, 2676. [Google Scholar] [CrossRef]
- Pullar, R.C.; Npvias, R.M.; Caetano, A.P.F.; Barreiros, M.A.; Abanades, S.; Costa Oliveira, F.A. A Review of Solar Thermochemical CO2 splitting using ceria-Based Ceramics With Designed Morphologies and Microstructures. Front. Chem. 2019, 7, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, L.R.; Chen, R.; Chen, X.; Chang, K.; Liu, Z.; Senanayake, S.D.; Ebrahim, A.M.; Chen, J.G. Elucidating the roles of metallic Ni and oxygen vacancies in CO2 hydrogenation over Ni/CeO2 using isotope exchange and in situ measurements. Appl. Catal. B Environ. 2019, 245, 360–366. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of promoted nickel catalysts supported on mesoporous nanocrystalline gamma alumina for carbon dioxide methanation reaction. J. Ind. Eng. Chem. 2014, 20, 4176–4182. [Google Scholar] [CrossRef]
- Solsona, B.; Sanchis, R.; Dejoz, A.M.; García, T.; Ruiz-Rodríguez, L.; López Nieto, J.M.; Cecilia, J.A.; Rodríguez-Castellón, E. Total Oxidation of Propane Using CeO2 and CuO-CeO2 Catalysts Prepared Using Templates of Different Nature. Catalysts 2017, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.R.; Sahu, H.R. XRD and UV-Vis diffuse reflectance analysis of CeO2–ZrO2 solid solutions synthesized by combustion method. J. Chem. Sci. 2001, 113, 651–658. [Google Scholar]
- Patsalas, P.; Logothetidis, S.; Sygellou, L.; Kennou, S. Structure Dependent Electronic Properties of Nanocrystalline Cerium Oxide Films. Phys. Rev. B 2003, 68, 035104. [Google Scholar] [CrossRef]
- Filtschew, A.; Hofmann, K.; Hess, C. Ceria and Its Defect Structure: New Insights from a Combined Spectroscopic Approach. J. Phys. Chem. C 2016, 120, 6694–6703. [Google Scholar] [CrossRef]
- Kainbayev, N.; Sriubas, M.; Virbukas, D.; Rutkuniene, Z.; Bockute, K.; Bolegenova, S.; Laukaitis, G. Raman Study of Nanocrystalline-Doped Ceria Oxide Thin Films. Coatings 2020, 10, 432. [Google Scholar] [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Garcia, X.; Soler, L.; Casanovas, A.; Escudero, C.; Llorca, J. X-ray photoelectron and Raman spectroscopy of nanostructured ceria in soot oxidation under operando conditions. Carbon 2021, 178, 164–180. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. CeO2-based catalysts with engineered morphologies for soot oxidation to enhance soot-catalyst contact. Nanoscale Res. Lett. 2014, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Soler, L.; Casanovas, A.; Urrich, A.; Angurell, I.; Llorca, J. CO oxidation and COPrOx over preformed Au nanoparticles supported over nanoshaped CeO2. Appl. Catal. B Environ. 2016, 197, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Sarli, V.; Landi, G.; Benedetto, A.; Lisi, L. Synergy Between Ceria and Metals (Ag or Cu) in Catalytic Diesel Particulate Filters: Efect of the Metal Content and of the Preparation Method on the Regeneration Performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Peymani, M.; Alavi, S.M.; Arandiyan, H.; Rezaei, M. Rational Design of High Durface Area mesoporous Ni/CeO2 for Partial Oxidation of Propane. Catalysts 2018, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Capel Schanez, M.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Strucutre and Surface properties of ceria- modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 5, 340–353. [Google Scholar] [CrossRef]
- Rakshit, S.; Ghosh, S.; Chall, S.; Mati, S.S.; Moulik, S.P.; Bhattacharya, S.C. Controlled synthesis of spin glass nickel oxide nanoparticles and evaluation of their potential antimicrobial: A cost effective and eco friendly approach. RSC Adv. 2013, 3, 19348. [Google Scholar] [CrossRef]
- Liu, X.; Han, L.; Liu, W.; Yang, Y. Synthesis of Co/Ni Unitary-or Binary-Doped CeO2 mesoporous Nanospheres and Their Catalytic Performance for CO Oxidation. Eur. J. Inorg. Chem. 2014, 28, 742–746. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, G.; Guo, Y.; Wang, Y.; Guo, Y. Effect of water vapor on the CO and CH4 catalytic oxidation over CeO2-MOx (M=Cu, Mn, Fe, Co, and Ni) mixed oxide. J. Rare Earths 2010, 28, 742–746. [Google Scholar] [CrossRef]
- Guan, J.; Mou, F.; Sun, Z.; Shi, W. Preparation of hollow spheres with controllable interior structures by heterogeneous contraction. Chem. Commun. 2010, 46, 6605–6607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.; Liu, Y.; Chen, Y.; Yang, S. Influence of preparation method on performance of Ni–CeO2 catalysts for reverse water-gas shift reaction. J. Rare Earths 2013, 31, 559–564. [Google Scholar] [CrossRef]
- Peymani, M.; Alavi, S.M.; Rezaei, M. Preparation of highly active and stable nanostructured Ni/CeO2 catalysts for syngas production by partial oxidation of methane. Int. J. Hydrogen Energy 2016, 41, 6316–6325. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Ramirez Reina, T.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A.; Sepúlveda-Escribano, A. Ni-CeO2/C catalysts with enhanced OSC for the WGS reaction. Catalysts 2015, 5, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Megías-Sayago, C.; Bonincontro, D.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Odriozola, J.A. 5-Hydroxymethyl-2-furfural oxidation over Au/CexZr1−xO2 catalysts. Front. Chem. 2020, 8, 461. [Google Scholar] [CrossRef]
- Gurbani, A.; Ayastuy, J.L.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. CuO–CeO2 catalysts synthesized by various methods: Comparative study of redox properties. Int. J. Hydrogen Energy 2010, 35, 11582–11590. [Google Scholar] [CrossRef]
- Gurbani, A.; Ayastuy, J.L.; González-Marcos, M.P.; Herrero, J.E.; Guil, J.M.; Gutiérrez-Ortiz, M.A. Comparative study of CuO–CeO2 catalysts prepared by wet impregnation and deposition–precipitation. Int. J. Hydrogen Energy 2009, 34, 547–553. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Ayastuy, J.L.; Boukha, Z.; Bravo, R.; Gutierrez-Ortiz, M.Á. Transition metals supported on bone-derived hydroxyapatite as potential catalysts for the Water-Gas Shift reaction. Renew. Energy 2018, 115, 641–648. [Google Scholar] [CrossRef]
- Boukha, Z.; González-Velasco, J.R.; Gutiérrez-Ortiz, M.A. Platinum supported on lanthana-modified hydroxyapatite samples for realistic WGS conditions: On the nature of the active species, kinetic aspects and the resistance to shut-down/start-up cycles. Appl. Catal. B Environ. 2020, 270, 118851. [Google Scholar] [CrossRef]
- Bensouilah, R.; Olivet, L.; Hammedi, T.; Pronier, S.; Barbier, J.; Fontaine, C.; Ghorbel, A.; Ksibi, Z. Effect of the variation of metal and cerium loadings on CeO2x–TiO2(100−x) supports in the complete catalytic oxidation of formaldehyde. Res. Chem. Intermed. 2021, 47, 813–834. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Bader, G.; Alemany, L.; Mariño, F. Pelletized Cu-Ni/CePr5 catalysts for H2 purification via water gas shift reaction. Fuel 2020, 271, 117653. [Google Scholar] [CrossRef]
- Boukha, Z.; Ayastuy, J.L.; González-Velasco, J.R.; Gutiérrez-Ortiz, M.A. CO elimination processes over promoter-free hydroxyapatite supported palladium catalysts. Appl. Catal. B Environ. 2017, 201, 189–201. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Sreńscek-Nazzal, J.; Llorca, J. Photocatalytic hydrogen production from alcohol aqueous solutions over TiO2-activated carbon composites decorated with Au and Pt. J. Photochem. Photobiol. A Chem. 2022, 425, 113726. [Google Scholar] [CrossRef]
- Serafin, J.; Kusiak-Nejman, E.; Wanag, A.; Morawski, A.W.; Llorca, J. Hydrogen photoproduction on TiO2-reduced graphene oxide hybrid materials from water-ethanol mixture. J. Photochem. Photobiol. A Chem. 2021, 418, 113406. [Google Scholar] [CrossRef]
- Stokes, D.J. Principles and Practice of Variable Pressure Environmental Scanning Electron Microscopy (VP-ESEM); John Wiley & Sons: Chichester, UK, 2008; ISBN 978-0470758748. [Google Scholar]














| Catalyst | SBET [m2 g−1] | Vtot [cm3 g−1] | VmN2 [cm3 g−1] | VmCO2 [cm3 g−1] |
|---|---|---|---|---|
| CeO2 polycrystalline | 63.7 | 0.1611 | 0.145 | 0.013 |
| CeO2 Rods | 18.8 | 0.1060 | 0.086 | 0.002 |
| CeO2 Cubes | 28.9 | 0.2832 | 0.129 | 0.006 |
| CeO2 Octahedra | 6.6 | 0.0573 | 0.019 | 0.001 |
| Sample | Crystallite Size Calculated Using XRD (nm) | Particle Size Calculated Using RAMAN (nm) | Particle Size Calculated Using SEM (nm) | Band Gap (eV) |
|---|---|---|---|---|
| CeO2 polycrystalline | 12.7 | 13.1 | 12–13 | 2.44 |
| CeO2 rods | 28.2 | 27.5 | 26–27 | 3.04 |
| CeO2 cubes | 14.3 | 16.1 | 15–16 | 2.60 |
| CeO2 octahedra | 21.1 | 20.5 | 20–21 | 2.69 |
| Catalyst | H2 Consumption [µmol/gcat] | CO2 Consumption [µmol/gcat] |
|---|---|---|
| CeO2 polycrystalline | 107.1 | 106.8 |
| CeO2 rods | 170.1 | 169.1 |
| CeO2 cubes | 124.9 | 124.4 |
| CeO2 octahedra | 20.3 | 20.1 |
| Sample | SBET (m2 g−1) | Vtot (cm3 g−1) | VmN2 (cm3 g−1) | Dp (nm) | Ni (wt.%) Loading According to XRF | Ni Dispersion (%) |
|---|---|---|---|---|---|---|
| 0.5%Ni-CeO2 rods | 18.7 | 0.14 | 0.018 | 12.4 | 0.44 | 4.2 |
| 1%Ni-CeO2 rods | 18.2 | 0.13 | 0.015 | 12.3 | 1.05 | 5.4 |
| 2%Ni-CeO2 rods | 17.1 | 0.12 | 0.015 | 12.3 | 2.09 | 9.7 |
| 5%Ni-CeO2 rods | 15 | 0.10 | 0.010 | 12.1 | 5.11 | 3.9 |
| 10%Ni-CeO2 rods | 10.5 | 0.05 | 0.005 | 11.9 | 9.86 | 2.5 |
| Catalyst | H2 Consumption [µmol/gcat] | CO2 Consumption [µmol/gcat] |
|---|---|---|
| CeO2 rods | 170.1 | 169.1 |
| 0.5%-Ni-CeO2 rods | 179.2 | 176.8 |
| 1%-Ni-CeO2 rods | 352.4 | 229.1 |
| 2%-Ni-CeO2 rods | 453.2 | 412 |
| 5%-Ni-CeO2 rods | 680.1 | 163.8 |
| 10%-Ni-CeO2 rods | 811.6 | 105.3 |
| Catalyst | OSC [μmol CO2/g] | Reference |
|---|---|---|
| CeO2 | 10 | [47] |
| CeO2/C | 255 | [45] |
| Ni-CeO2/C | 850 | [45] |
| AuCe | 103.03 | [48] |
| AuCe50Zr | 332.68 | [48] |
| AuCe25Zr | 124.88 | [48] |
| CuCe (solegel method) | 107 | [49] |
| CuCe (wet impregnation) | 476 | [50] |
| Ni/hydroxyapatite | 58.80 | [51] |
| Pt/hydroxyapatite | 45 | [52] |
| CeO2-TiO2 | 66 | [53] |
| Ni/CePr5/Al-1/4 | 233 | [54] |
| Pt/La(8)/hydroxyapatite | 77 | [52] |
| Pd(0.5)/hydroxyapatite | 64.2 | [55] |
| 2%-Ni-CeO2-rods | 412 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafin, J.; Llorca, J. Nanoshaped Cerium Oxide with Nickel as a Non-Noble Metal Catalyst for CO2 Thermochemical Reactions. Molecules 2023, 28, 2926. https://doi.org/10.3390/molecules28072926
Serafin J, Llorca J. Nanoshaped Cerium Oxide with Nickel as a Non-Noble Metal Catalyst for CO2 Thermochemical Reactions. Molecules. 2023; 28(7):2926. https://doi.org/10.3390/molecules28072926
Chicago/Turabian StyleSerafin, Jarosław, and Jordi Llorca. 2023. "Nanoshaped Cerium Oxide with Nickel as a Non-Noble Metal Catalyst for CO2 Thermochemical Reactions" Molecules 28, no. 7: 2926. https://doi.org/10.3390/molecules28072926
APA StyleSerafin, J., & Llorca, J. (2023). Nanoshaped Cerium Oxide with Nickel as a Non-Noble Metal Catalyst for CO2 Thermochemical Reactions. Molecules, 28(7), 2926. https://doi.org/10.3390/molecules28072926