Effect of Metal Complexing on Mn–Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Patterns
2.2. FT−IR Spectroscopy
2.3. N2 Adsorption–Desorption
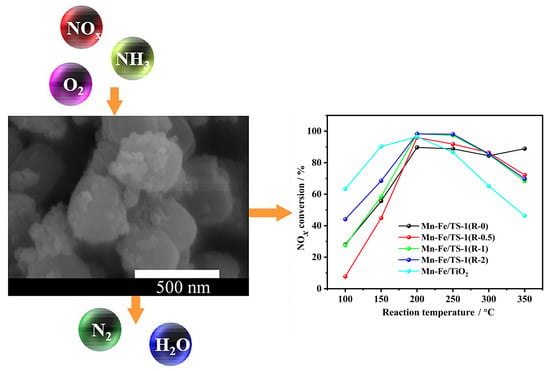
2.4. SEM Images and EDS Analysis
2.5. XPS Analysis
2.6. H2-TPR
2.7. NH3-TPD
2.8. NH3-SCR Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B-Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Zhang, T.T.; Yan, L.M. Enhanced low-temperature NH3-SCR performance of Ce/TiO2 modified by Ho catalyst. Roy. Soc. Open Sci. 2019, 6, 182120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Guo, J.X.; Shi, X.K.; Wen, X.R.; Chu, Y.H.; Yuan, S.D. Effect of aluminum on the catalytic performance and reaction mechanism of Mn/MCM-41 for NH3-SCR reaction. Appl. Surf. Sci. 2020, 534, 147592. [Google Scholar] [CrossRef]
- Pu, Y.J.; Yang, L.; Yao, C.; Jiang, W.J.; Yao, L. Low-cost Mn-Fe/SAPO-34 catalyst from natural ferromanganese ore and lithium-silicon-powder waste for efficient low-temperature NH3-SCR removal of NOx. Chemosphere 2022, 293, 133465. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Lu, T.; Cheng, K.; Xu, H.M. Performance of V2O5-WO3-MoO3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, H.Y.; Zhong, Q. Promotional effect of F-doped V2O5-WO3/TiO2 catalyst for NH3-SCR of NO at low-temperature. Appl. Catal. A-Gen. 2012, 435, 156–162. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Zhang, L.; Huang, L.; Li, H.; Gao, R.; Shi, L.; Zhang, J. Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2−δ (M = Fe, Cu, Mn, Co) for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2014, 4, 93–101. [Google Scholar] [CrossRef]
- Zhang, N.; He, H.; Wang, D.; Li, Y. Challenges and opportunities for manganese oxides in low-temperature selective catalytic reduction of NOx with NH3: H2O resistance ability. J. Solid State Chem. 2020, 289, 121464. [Google Scholar] [CrossRef]
- Zhang, B.L.; Liebau, M.; Suprun, W.; Liu, B.; Zhang, S.G.; Glaser, R. Suppression of N2O formation by H2O and SO2 in the selective catalytic reduction of NO with NH3 over a Mn/Ti-Si catalyst. Catal. Sci. Technol. 2019, 9, 4759–4770. [Google Scholar] [CrossRef]
- Park, T.S.; Jeong, S.K.; Hong, S.H.; Hong, S.C. Selective catalytic reduction of nitrogen oxides with NH3 over natural manganese ore at low temperature. Ind. Eng. Chem. Res. 2001, 40, 4491–4495. [Google Scholar] [CrossRef]
- Guo, R.-t.; Sun, X.; Liu, J.; Pan, W.-g.; Li, M.-y.; Liu, S.-m.; Sun, P.; Liu, S.-w. Enhancement of the NH3-SCR catalytic activity of MnTiOx catalyst by the introduction of Sb. Appl. Catal. A-Gen. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Wu, S.G.; Zhang, L.; Wang, X.B.; Zou, W.X.; Cao, Y.; Sun, J.F.; Tang, C.J.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A-Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Li, L.; Ji, J.; Tan, W.; Song, W.; Wang, X.; Wei, X.; Guo, K.; Zhang, W.; Tang, C.; Dong, L. Enhancing low-temperature NH3-SCR performance of Fe–Mn/CeO2 catalyst by Al2O3 modification. J. Rare. Earth. 2022, 40, 1454–1461. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Byproduct Analysis of SO2 Poisoning on NH3-SCR over MnFe/TiO2 Catalysts at Medium to Low Temperatures. Catalysts. 2019, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, B.; Wang, H.; Ma, J.; Xu, W.Q.; Li, Y.; Han, Y.; Sun, Q. Fe and/or Mn oxides supported on fly ash-derived SBA-15 for low-temperature NH3-SCR. Catal. Commun. 2018, 108, 82–87. [Google Scholar] [CrossRef]
- Mu, J.C.; Li, X.Y.; Sun, W.B.; Fan, S.Y.; Wang, X.Y.; Wang, L.; Qin, M.C.; Gan, G.Q.; Yin, Z.F.; Zhang, D.K. Enhancement of Low-Temperature Catalytic Activity over a Highly Dispersed Fe-Mn/Ti Catalyst for Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Hou, X.X.; Chen, H.P.; Liang, Y.H.; Wei, Y.L.; Li, Z.Q. La Modified Fe-Mn/TiO2 Catalysts to Improve SO2 Resistance for NH3-SCR at Low-Temperature. Catal. Surv. Asia 2020, 24, 291–299. [Google Scholar] [CrossRef]
- Xu, G.Y.; Guo, X.L.; Cheng, X.X.; Yu, J.; Fang, B.Z. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 2021, 13, 7052–7080. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Zhu, J.; Ma, J.; Qin, Q. A review on titanosilicate-1 (TS-1) catalysts: Research progress of regulating titanium species. Coordin. Chem. Rev. 2023, 476, 214931. [Google Scholar] [CrossRef]
- Gu, J.L.; Duan, R.D.; Chen, W.B.; Chen, Y.; Liu, L.L.; Wang, X.D. Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction. Catalysts 2020, 10, 566. [Google Scholar] [CrossRef]
- Niu, C.; Shi, X.; Liu, F.; Liu, K.; Xie, L.; You, Y.; He, H. High hydrothermal stability of Cu–SAPO-34 catalysts for the NH3-SCR of NOx. Chem. Eng. J. 2016, 294, 254–263. [Google Scholar] [CrossRef]
- Wen, C.; Wang, X.; Xu, J.; Fan, Y. Hierarchical SAPO-11 molecular sieve-based catalysts for enhancing the double-branched hydroisomerization of alkanes. Fuel 2019, 255, 115821. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.; Xu, C.; Liu, J.; Zhao, Z.; Wei, Y.; Duan, A.; Jiang, G. The selective catalytic reduction of NOx over a Cu/ZSM-5/SAPO-34 composite catalyst. RSC Adv. 2015, 5, 104923–104931. [Google Scholar] [CrossRef]
- Feng, X.; Sheng, N.; Liu, Y.; Chen, X.; Chen, D.; Yang, C.; Zhou, X. Simultaneously Enhanced Stability and Selectivity for Propene Epoxidation with H2 and O2 on Au Catalysts Supported on Nano-Crystalline Mesoporous TS-1. ACS Catal. 2017, 7, 2668–2675. [Google Scholar] [CrossRef]
- Yue, M.B.; Sun, M.N.; Xie, F.; Ren, D.D. Dry-gel synthesis of hierarchical TS-1 zeolite by using P123 and polyurethane foam as template. Micropor. Mesopor. Mat. 2014, 183, 177–184. [Google Scholar] [CrossRef]
- Xue, T.; Liu, H.; Wang, Y.; Wu, H.; Wu, P.; He, M. Seed-induced synthesis of small-crystal TS-1 using ammonia as alkali source. Chinese J. Catal. 2015, 36, 1928–1935. [Google Scholar] [CrossRef]
- Raveendra, G.; Li, C.; Cheng, Y.; Meng, F.; Li, Z. Direct transformation of syngas to lower olefins synthesis over hybrid Zn–Al2O3/SAPO-34 catalysts. New. J. Chem. 2018, 42, 4419–4431. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Peng, H.; Mi, Y.; Liang, J.; Liu, W.; Wang, X.; Song, G.; Wu, P.; Liu, F. One-pot synthesis of layered mesoporous ZSM-5 plus Cu ion-exchange: Enhanced NH3-SCR performance on Cu-ZSM-5 with hierarchical pore structures. J. Hazard. Mater. 2020, 385, 121593. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Guo, Z.; Yang, H.; Shao, J.; Zhang, X.; Zhang, S. One-pot hydrothermal synthesis of dual metal incorporated CuCe-SAPO-34 zeolite for enhancing ammonia selective catalytic reduction. J. Hazard. Mater. 2021, 405, 124177. [Google Scholar] [CrossRef]
- Yan, R.; Lin, S.; Li, Y.; Liu, W.; Mi, Y.; Tang, C.; Wang, L.; Wu, P.; Peng, H. Novel shielding and synergy effects of Mn-Ce oxides confined in mesoporous zeolite for low temperature selective catalytic reduction of NOx with enhanced SO2/H2O tolerance. J. Hazard. Mater. 2020, 396, 122592. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, T.; Dang, H.; Chen, X.; You, Y.; Schwank, J.W.; Li, J. Fe2O3@SiTi core–shell catalyst for the selective catalytic reduction of NOx with NH3: Activity improvement and HCl tolerance. Catal. Sci. Technol. 2018, 8, 3313–3320. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5O mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance. Appl. Catal. B-Environ. 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, S.; Jiang, W.; Feng, W.; Jiang, H. Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-Y.; Lee, C.-Y.; Zhang, Y.-R.; Bai, H. Aerosol-assisted deposition of Mn-Fe oxide catalyst on TiO2 for superior selective catalytic reduction of NO with NH3 at low temperatures. Catal. Commun. 2018, 111, 36–41. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx–CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Metal Sulfate Poisoning Effects over MnFe/TiO2 for Selective Catalytic Reduction of NO by NH3 at Low Temperature. Ind. Eng. Chem. Res. 2018, 57, 4848–4858. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, D.; Li, B.; Wang, C.; Li, J.; Crittenden, J.; Hao, J. Impacts of Pb and SO2 Poisoning on CeO2–WO3/TiO2–SiO2 SCR Catalyst. Environ. Sci. Technol. 2017, 51, 11943–11949. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Kamasamudram, K.; Epling, W.S. In Situ-DRIFTS Study of Selective Catalytic Reduction of NOx by NH3 over Cu-Exchanged SAPO-34. ACS Catal. 2013, 3, 871–881. [Google Scholar] [CrossRef]
- Gong, J.; Narayanaswamy, K.; Rutland, C.J. Heterogeneous Ammonia Storage Model for NH3–SCR Modeling. Ind. Eng. Chem. Res. 2016, 55, 5874–5884. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D.J.C.R. Selective catalytic reduction of NO x with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Liang, J.; Mi, Y.; Song, G.; Peng, H.; Li, Y.; Yan, R.; Liu, W.; Wang, Z.; Wu, P.; Liu, F. Environmental benign synthesis of Nano-SSZ-13 via FAU trans-crystallization: Enhanced NH3-SCR performance on Cu-SSZ-13 with nano-size effect. J. Hazard. Mater. 2020, 398, 122986. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wen, C.; Wu, Z.; Wang, J.; Chang, L.; Feng, G.; Zhang, R.; Kong, D.; Liu, J. Density functional theory investigations into the structures and acidity properties of Ti-doped SSZ-13 zeolite. Micropor. Mesopor. Mat. 2017, 237, 132–139. [Google Scholar] [CrossRef]








| Samples | SBET a (m2/g) | Smeso b (m2/g) | Vtotal c (cm3/g) | Vmicro b (cm3/g) | Vmeso b (cm3/g) | Mn d (wt %) | Fe d (wt %) |
|---|---|---|---|---|---|---|---|
| Mn–Fe/TS-1(R-0) | 323 | 85 | 0.24 | 0.10 | 0.14 | 3.4 | 2.1 |
| Mn–Fe/TS-1(R-0.5) | 311 | 83 | 0.27 | 0.10 | 0.17 | 2.5 | 1.7 |
| Mn–Fe/TS-1(R-1) | 317 | 82 | 0.31 | 0.10 | 0.21 | 2.0 | 1.2 |
| Mn–Fe/TS-1(R-2) | 306 | 74 | 0.26 | 0.10 | 0.16 | 3.9 | 4.9 |
| Mn–Fe/TiO2 | -- | -- | -- | -- | -- | 13.5 | 17.9 |
| Samples | Atomic Concentration | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| Mn (at. %) | Fe (at. %) | Ti (at. %) | Mn4+/Mnsuf (%) | Fe2+/Fesuf (%) | Oα/Osuf (%) | |
| Mn–Fe/TS-1(R-0) | 2.28 | 6.96 | 2.58 | 12.5 | 8.71 | 13.0 |
| Mn–Fe/TS-1(R-0.5) | 1.46 | 2.7 | 1.52 | 18.7 | 9.18 | 4.7 |
| Mn–Fe/TS-1(R-1) | 1.88 | 3.58 | 1.84 | 7.3 | 13.31 | 7.9 |
| Mn–Fe/TS-1(R-2) | 0.21 | 3.43 | 1.41 | 16.1 | 15.88 | 8.6 |
| Mn–Fe/TiO2 | 2.43 | 0.31 | 22.32 | 11.4 | 15.55 | 7.06 |
| Samples | Temperature (°C)/H2 Consumption (mL·g−1, STP) | ||||
|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Total | |
| Mn-Fe/TS-1(R-0) | 439/46.72 | 534/16.70 | 598/19.26 | --/-- | --/82.69 |
| Mn-Fe/TS-1(R-0.5) | 424/42.61 | 554/6.15 | 655/23.44 | --/-- | --/72.22 |
| Mn-Fe/TS-1(R-1) | 436/5.79 | 552/36.02 | 665/5.10 | --/-- | --/46.91 |
| Mn-Fe/TS-1(R-2) | 437/69.74 | 611/22.46 | 687/39.23 | --/-- | --/131.43 |
| Mn-Fe/TiO2 | 281/22.38 | 363/15.08 | 502/7.63 | 582/1.23 | --/46.32 |
| Samples | Temperature (°C)/NH3 Adsorption Amount (mL·g−1, STP) | |||
|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Total | |
| Mn–Fe/TS-1(R-0) | 146/0.10 | 314/0.15 | --/-- | --/0.25 |
| Mn–Fe/TS-1(R-0.5) | 136/0.07 | 223/0.11 | --/-- | --/0.18 |
| Mn–Fe/TS-1(R-1) | 157/0.09 | 262/0.12 | --/-- | --/0.21 |
| Mn–Fe/TS-1(R-2) | 151/0.06 | 267/0.20 | --/-- | --/0.26 |
| Mn–Fe/TiO2 | 193/0.07 | 266/0.16 | 518/0.10 | --/0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, W.; Li, Z.; Sun, Y.; Shi, M.; Nan, Z.; Song, R.; Wang, L.; Guan, J. Effect of Metal Complexing on Mn–Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3. Molecules 2023, 28, 3068. https://doi.org/10.3390/molecules28073068
Ma Y, Liu W, Li Z, Sun Y, Shi M, Nan Z, Song R, Wang L, Guan J. Effect of Metal Complexing on Mn–Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3. Molecules. 2023; 28(7):3068. https://doi.org/10.3390/molecules28073068
Chicago/Turabian StyleMa, Yuanyuan, Wanting Liu, Zhifang Li, Yuhang Sun, Mingyuan Shi, Zheng Nan, Ruotong Song, Liying Wang, and Jingqi Guan. 2023. "Effect of Metal Complexing on Mn–Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3" Molecules 28, no. 7: 3068. https://doi.org/10.3390/molecules28073068
APA StyleMa, Y., Liu, W., Li, Z., Sun, Y., Shi, M., Nan, Z., Song, R., Wang, L., & Guan, J. (2023). Effect of Metal Complexing on Mn–Fe/TS-1 Catalysts for Selective Catalytic Reduction of NO with NH3. Molecules, 28(7), 3068. https://doi.org/10.3390/molecules28073068