Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds
Abstract
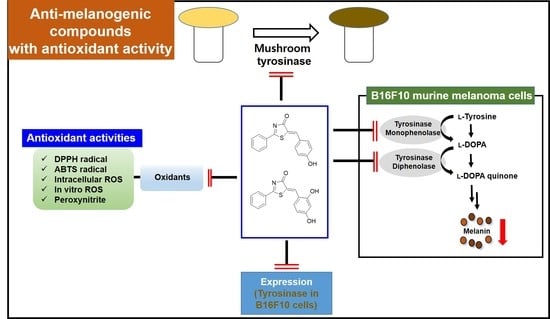
:1. Introduction
2. Results and Discussion
2.1. Synthesis of (Z)-BPT Derivatives 1–14
2.2. Tyrosinase Inhibition of (Z)-BPT Derivatives
2.3. Identifying the Inhibition Type of (Z)-BPT Derivatives 1–3
2.4. In Silico Docking of (Z)-BPT Derivatives 1–3 with Mushroom Tyrosinase
2.5. In Silico Docking of (Z)-BPT Derivatives 1–3 with the Human Tyrosinase Model
2.6. Effects of (Z)-BPT Derivatives 1–3 on B16F10 Cell Viability
2.7. Effects of (Z)-BPT Derivatives 1 and 2 on Melanin Production in B16F10 Cells
2.8. Effects of (Z)-BPT Derivatives 1 and 2 on B16F10 Cellular Tyrosinase Activity
2.9. ABTS Cation Radical Scavenging Effects of (Z)-BPT Derivatives 1–14
2.10. DPPH Radical Scavenging Effects of (Z)-BPT Derivatives 1–14
2.11. ROS Scavenging Effects of (Z)-BPT Derivatives 1–14
2.12. Peroxynitrite (ONOO−) Scavenging Effects of (Z)-BPT Derivatives 1–14
2.13. Effects of (Z)-BPT Derivatives 1–2 on Tyrosinase Expression
3. Materials and Methods
3.1. Reagents
3.2. Chemistry
3.2.1. General Methods
3.2.2. General Procedure for the Synthesis of (Z)-BPT Derivatives 1–14
3.2.3. Procedure for the Synthesis of Compound 16 [43]
3.3. Kinetic and In Silico Studies and In Vitro Assays
3.3.1. Inhibition Assay against Mushroom Tyrosinase
3.3.2. Kinetic Studies on the Inhibition of Mushroom Tyrosinase by (Z)-BPT Derivatives 1–3
3.3.3. In Silico Study of Kojic Acid and (Z)-BPT Derivatives 1–3 on Mushroom Tyrosinase (mTYR)
3.3.4. In Silico Study of Kojic Acid and (Z)-BPT Derivatives 1–3 on Human Tyrosinase Homology Model
3.3.5. B16F10 Murine Melanoma Cell Culture
3.3.6. Cytotoxicity Analysis of (Z)-BPT Derivatives 1–3 in B16F10 Melanoma Cells
3.3.7. Anti-Melanogenesis Assay of Kojic Acid and (Z)-BPT Derivatives 1 and 2 in B16F10 Cells
3.3.8. Anti-Tyrosinase Activity Assay of Kojic Acid and (Z)-BPT Derivatives 1 and 2 in B16F10 Cells
3.3.9. ABTS Cation-Free Radical Scavenging Assay of (Z)-BPT Derivatives 1–14
3.3.10. DPPH Radical Scavenging Assay of (Z)-BPT Derivatives 1–14
3.3.11. Intracellular ROS Scavenging Activity Assay of (Z)-BPT Derivatives 1–14
3.3.12. In Vitro ROS Scavenging Activity Assay of (Z)-BPT Derivatives 1–14
3.3.13. Peroxynitrite (ONOO−) Scavenging Assay of (Z)-BPT Derivatives 1–14
3.3.14. Western Blot Assay of Tyrosinase Protein
3.3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Riaz, R.; Batool, S.; Zucca, P.; Rescigno, A.; Peddio, S.; Saleem, S.Z.R. Plants as a Promising Reservoir of Tyrosinase Inhibitors. Mini-Rev. Org. Chem. 2021, 18, 808–828. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Derm. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Ali, S.A.; Choudhary, R.; Naaz, I.; Ali, A.S. In Understanding the Challenges of Melanogenesis: Key Role of Bioactive Compounds in the Treatment of Hyperpigmentory Disorders. J. Pigment. Dis. 2015, 2, 1–9. [Google Scholar]
- Szabó, G.; Gerald, A.B.; Pathak, M.A.; Fitzpatrick, T.B. Racial differences in the fate of melanosomes in human epidermis. Nature 1969, 222, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Tankhaeva, L.M.; Rokhin, A.V.; Agafonova, S.V. Physicochemical properties and antioxidant activity of melanin fractions from Inonotus obliquus sclerotia. Chem. Nat. Compd. 2012, 48, 396–403. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological Properties of Melanin and its Function in Health. Basic Clin. Pharm. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger, N.; Rossowska, J.; Latajka, R. Inhibitory properties of aromatic thiosemicarbazones on mushroom tyrosinase: Synthesis, kinetic studies, molecular docking and effectiveness in melanogenesis inhibition. Bioorg. Chem. 2018, 81, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert Opin. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, H.J.; Choi, Y.J.; Park, Y.J.; Woo, Y.; Kim, S.J.; Park, M.H.; Lee, H.W.; Chun, P.; Chung, H.Y.; et al. Benzylidene-linked thiohydantoin derivatives as inhibitors of tyrosinase and melanogenesis: Importance of the β-phenyl-α,β-unsaturated carbonyl functionality. MedChemComm 2014, 5, 1410–1417. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Tien Guan Thng, S.; Kumar Verma, N.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.M.; Emami, S. Kojic acid-derived tyrosinase inhibitors: Synthesis and bioactivity. Pharm. Biomed. Res. 2015, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and Involvement of Aqueous Reducing Compounds in Oxidative Defense Systems of Various Senescing Leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.-g. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef]
- Lee, S.; Ullah, S.; Park, C.; Won Lee, H.; Kang, D.; Yang, J.; Akter, J.; Park, Y.; Chun, P.; Moon, H.R. Inhibitory effects of N-(acryloyl)benzamide derivatives on tyrosinase and melanogenesis. Bioorg. Med. Chem. 2019, 27, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Choi, D.C.; Noh, S.G.; Choi, H.; Choi, I.; Ryu, I.Y.; Chung, H.Y.; Moon, H.R. New Benzimidazothiazolone Derivatives as Tyrosinase Inhibitors with Potential Anti-Melanogenesis and Reactive Oxygen Species Scavenging Activities. Antioxidants 2021, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Park, Y.; Ryu, I.Y.; Jung, H.J.; Ullah, S.; Choi, H.; Park, C.; Kang, D.; Lee, S.; Chun, P.; et al. In silico and in vitro insights into tyrosinase inhibitors with a 2-thioxooxazoline-4-one template. Comput. Struct. Biotechnol. J. 2021, 19, 37–50. [Google Scholar] [CrossRef]
- Ryu, I.Y.; Choi, I.; Jung, H.J.; Ullah, S.; Choi, H.; Al-Amin, M.; Chun, P.; Moon, H.R. In vitro anti-melanogenic effects of chimeric compounds, 2-(substituted benzylidene)-1,3-indanedione derivatives with a β-phenyl-α, β -unsaturated dicarbonyl scaffold. Bioorg. Chem. 2021, 109, 104688. [Google Scholar] [CrossRef]
- Choi, H.; Young Ryu, I.; Choi, I.; Ullah, S.; Jin Jung, H.; Park, Y.; Hwang, Y.; Jeong, Y.; Hong, S.; Chun, P.; et al. Identification of (Z)-2-benzylidene-dihydroimidazothiazolone derivatives as tyrosinase inhibitors: Anti-melanogenic effects and in silico studies. Comput. Struct. Biotechnol. J. 2022, 20, 899–912. [Google Scholar] [CrossRef]
- Ko, J.; Lee, J.; Jung, H.J.; Ullah, S.; Jeong, Y.; Hong, S.; Kang, M.K.; Park, Y.J.; Hwang, Y.; Kang, D.; et al. Design and Synthesis of (Z)-5-(Substituted benzylidene)-3-cyclohexyl-2-thioxothiazolidin-4-one Analogues as Anti-Tyrosinase and Antioxidant Compounds: In Vitro and In Silico Insights. Antioxidants 2022, 11, 1918. [Google Scholar] [CrossRef]
- Jeong, Y.; Hong, S.; Jung, H.J.; Ullah, S.; Hwang, Y.; Choi, H.; Ko, J.; Lee, J.; Chun, P.; Chung, H.Y.; et al. Identification of a Novel Class of Anti-Melanogenic Compounds, (Z)-5-(Substituted benzylidene)-3-phenyl-2-thioxothiazolidin-4-one Derivatives, and Their Reactive Oxygen Species Scavenging Activities. Antioxidants 2022, 11, 948. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.J.; Jung, H.J.; Ullah, S.; Yoon, D.; Jeong, Y.; Kim, G.Y.; Kang, M.K.; Kang, D.; Park, Y.; et al. Design and Synthesis of (Z)-2-(Benzylamino)-5-benzylidenethiazol-4(5H)-one Derivatives as Tyrosinase Inhibitors and Their Anti-Melanogenic and Antioxidant Effects. Molecules 2023, 28, 848. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, Y.M.; Moon, K.M.; Choi, Y.J.; Park, Y.J.; Jeong, H.O.; Chung, K.W.; Lee, H.J.; Chun, P.; Moon, H.R.; et al. Anti-melanogenic effect of (Z)-5-(2,4-dihydroxybenzylidene) thiazolidine-2,4-dione, a novel tyrosinase inhibitor. Arch. Pharm. Res. 2013, 36, 1189–1197. [Google Scholar] [CrossRef]
- Jung, H.J.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Moon, H.R. In vitro and in silico insights into tyrosinase inhibitors with (E)-benzylidene-1-indanone derivatives. Comput. Struct. Biotechnol. J. 2019, 17, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Petrauskas, A.A.; Kolovanov, E.A. ACD/Log P method description. Perspect. Drug Discov. Des. 2000, 19, 99–116. [Google Scholar] [CrossRef]
- Yang, K.-X.; Ji, D.-S.; Zheng, H.; Gu, Y.; Xu, P.-F. Organocatalytic inverse-electron-demand Diels–Alder reaction between 5-alkenyl thiazolones and β,γ-unsaturated carbonyl compounds. Chem. Commun. 2022, 58, 4227–4230. [Google Scholar] [CrossRef]
- Vögeli, U.; von Philipsborn, W.; Nagarajan, K.; Nair, M.D. Structures of Addition Products of Acetylenedicarboxylic Acid Esters with Various Dinucleophiles. An application of C, H-spin-coupling constants. Helv. Chim. Acta 1978, 61, 607–617. [Google Scholar] [CrossRef]
- Ullah, S.; Park, Y.; Ikram, M.; Lee, S.; Park, C.; Kang, D.; Yang, J.; Akter, J.; Yoon, S.; Chun, P.; et al. Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorg. Med. Chem. 2018, 26, 5672–5681. [Google Scholar] [CrossRef] [PubMed]
- Ashooriha, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Moradi, S.E.; Rafiei, A.; Kardan, M.; Emami, S. 1, 2, 3-Triazole-based kojic acid analogs as potent tyrosinase inhibitors: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 82, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mo, J.; Xiong, B.; Liao, Q.; Chen, Y.; Wang, Y.; Xing, S.; He, S.; Lyu, W.; Zhang, N.; et al. Discovery of Resorcinol-Based Polycyclic Structures as Tyrosinase Inhibitors for Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-C.; Rho, H.S.; Joo, Y.H.; Lee, C.S.; Lee, J.; Ahn, S.M.; Kim, J.E.; Shin, S.S.; Park, Y.-H.; Suh, K.-D.; et al. Depigmenting activities of kojic acid derivatives without tyrosinase inhibitory activities. Bioorg. Med. Chem. Lett. 2012, 22, 4159–4162. [Google Scholar] [CrossRef]
- Lu, T.M.; Ko, H.H.; Ng, L.T.; Hsieh, Y.P. Free-Radical-Scavenging, Antityrosinase, and Cellular Melanogenesis Inhibitory Activities of Synthetic Isoflavones. Chem Biodivers. 2015, 12, 963–979. [Google Scholar] [CrossRef]
- Gamal El-Din, M.I.; Youssef, F.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. New γ-pyrone glycoside from Pachira glabra and assessment of its gastroprotective activity using an alcohol-induced gastric ulcer model in rats. Food Funct. 2020, 11, 1958–1965. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.T.; Cooper, M.A.; Barnard, R.T.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharm. 2022, 13, 823881. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.F.; LeBel, C.P.; Bondy, S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 1992, 13, 637–648. [Google Scholar]
- Padmaja, S.; Madison, S. Reaction of peroxynitrite with the melanin precursor, 5, 6-dihydroxyindole-2-carboxylic acid. Res. Chem. Intermed. 1999, 25, 441–458. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein–Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef]
- Ha, Y.M.; Park, Y.J.; Kim, J.-A.; Park, D.; Park, J.Y.; Lee, H.J.; Lee, J.Y.; Moon, H.R.; Chung, H.Y. Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur. J. Med. Chem. 2012, 49, 245–252. [Google Scholar] [CrossRef]
- Lee, S.; Choi, H.; Park, Y.; Jung, H.J.; Ullah, S.; Choi, I.; Kang, D.; Park, C.; Ryu, I.Y.; Jeong, Y.; et al. Urolithin and Reduced Urolithin Derivatives as Potent Inhibitors of Tyrosinase and Melanogenesis: Importance of the 4-Substituted Resorcinol Moiety. Int. J. Mol. Sci. 2021, 22, 5616. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.J.; Ullah, S.; Yun, H.Y.; Chun, P.; Moon, H.R. Design, synthesis, and antimelanogenic effects of (2-substituted phenyl-1,3-dithiolan-4-yl)methanol derivatives. Drug Des. Devel. 2017, 11, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef] [PubMed]














| Cpd | R1 | R2 | R3 | R4 | IC50 (µM) | Synthetic Yield (%) | Log P |
|---|---|---|---|---|---|---|---|
| 1 | H | H | OH | H | 6.4 ± 0.52 | 81% | 3.55 |
| 2 | OH | H | OH | H | 0.1 ± 0.01 | 76% | 3.16 |
| 3 | H | OH | OH | H | 5.2 ± 0.32 | 72% | 3.16 |
| 4 | H | OMe | OH | H | 211.1 ± 1.84 | 72% | 3.42 |
| 5 | H | OEt | OH | H | 73.8 ± 2.02 | 54% | 3.76 |
| 6 | H | OH | OMe | H | 121.1 ± 2.72 | 69% | 3.42 |
| 7 | OH | H | H | H | >300 | 52% | 3.55 |
| 8 | H | H | OMe | H | >300 | 78% | 3.81 |
| 9 | OMe | H | OMe | H | >300 | 49% | 3.69 |
| 10 | H | OMe | OMe | H | >300 | 43% | 3.69 |
| 11 | H | OMe | OMe | OMe | >300 | 60% | 3.56 |
| 12 | H | OMe | OH | OMe | 109.0 ± 2.81 | 61% | 3.30 |
| 13 | H | Br | OH | H | 253.7 ± 1.87 | 72% | 4.38 |
| 14 | H | Br | OH | Br | 87.2 ± 0.63 | 41% | 5.21 |
| KA | 20.8 ± 1.34 | −2.45 | |||||
 | |||||||
| Compound | Inhibition Type a | Compound | Inhibition Type |
|---|---|---|---|
| 1 | Competitive | 3 | mixed |
| 2 | Competitive | Kojic acid | Mixed b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, D.; Kang, M.K.; Jung, H.J.; Ullah, S.; Lee, J.; Jeong, Y.; Noh, S.G.; Kang, D.; Park, Y.; Chun, P.; et al. Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds. Molecules 2023, 28, 3293. https://doi.org/10.3390/molecules28083293
Yoon D, Kang MK, Jung HJ, Ullah S, Lee J, Jeong Y, Noh SG, Kang D, Park Y, Chun P, et al. Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds. Molecules. 2023; 28(8):3293. https://doi.org/10.3390/molecules28083293
Chicago/Turabian StyleYoon, Dahye, Min Kyung Kang, Hee Jin Jung, Sultan Ullah, Jieun Lee, Yeongmu Jeong, Sang Gyun Noh, Dongwan Kang, Yujin Park, Pusoon Chun, and et al. 2023. "Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds" Molecules 28, no. 8: 3293. https://doi.org/10.3390/molecules28083293
APA StyleYoon, D., Kang, M. K., Jung, H. J., Ullah, S., Lee, J., Jeong, Y., Noh, S. G., Kang, D., Park, Y., Chun, P., Chung, H. Y., & Moon, H. R. (2023). Design, Synthesis, In Vitro, and In Silico Insights of 5-(Substituted benzylidene)-2-phenylthiazol-4(5H)-one Derivatives: A Novel Class of Anti-Melanogenic Compounds. Molecules, 28(8), 3293. https://doi.org/10.3390/molecules28083293