Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review
Abstract
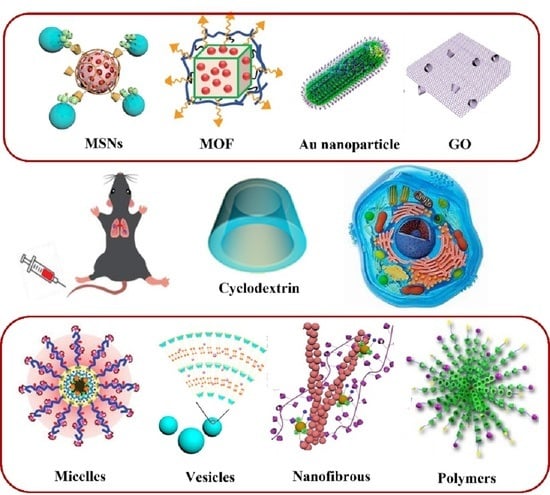
:1. Introduction
2. Cyclodextrins
3. Cyclodextrins’ Advantage in Cancer Theranostics
4. Biomedical Applications
4.1. Bioimaging and Molecular Recognition
4.2. Nanodevices for Controlled Drug Delivery
4.3. Nanodevices for Gene and Protein Delivery
4.4. Photodynamic Therapy (PDT)
4.5. Photothermal Therapy (PTT)
5. Deficiencies of Current CD-Mediated Cancer Theranostics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.C.; West, C.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coley, H.M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008, 34, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- James, T.D. Specialty Grand Challenges in Supramolecular Chemistry. Front. Chem. 2017, 5, 83. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-W.; Sun, Y.; Song, N. Switchable Host–Guest Systems on Surfaces. Acc. Chem. Res. 2014, 47, 1950–1960. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893, Correction in Chem. Soc. Rev. 2014, 43, 7236. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Xu, J.-F.; Chen, L.; Zhang, X. How to Make Weak Noncovalent Interactions Stronger. Chem. A Eur. J. 2015, 21, 11938–11946. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Q.; Peng, X.; Zhang, H.; Zeng, H. Probing and Manipulating Noncovalent Interactions in Functional Polymeric Systems. Chem. Rev. 2022, 122, 14594–14678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Khalil-Cruz, L.E.; Khashab, N.M.; Yu, G.; Huang, F. Pillararene-based supramolecular systems for theranostics and bioapplications. Sci. China Chem. 2021, 64, 688–700. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2019, 32, e1806158. [Google Scholar] [CrossRef]
- Yuan, Y.; Nie, T.; Fang, Y.; You, X.; Huang, H.; Wu, J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J. Mater. Chem. B 2022, 10, 2077–2096. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Zhu, H.; Shangguan, L.; Shi, B.; Yu, G.; Huang, F. Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles. Mater. Chem. Front. 2018, 2, 2152–2174. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y. Multicharged cyclodextrin supramolecular assemblies. Chem. Soc. Rev. 2022, 51, 4786–4827. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef]
- Deng, B.; Yang, J.; Guo, M.; Yang, R. Highly efficient catalytic performance on CuAAC reaction by polymer-like supramolecular self-assemblies-Cu(I) in aqueous solution. Appl. Organomet. Chem. 2022, 36, e6674. [Google Scholar] [CrossRef]
- Yan, Y.-X.; Zhang, Y.; Chen, Y.; Liu, Y. Cyclodextrin-Based Supramolecular Hydrogel as a Selective Chiral Adsorption/Separation Platform for Tryptophan Enantiomers. ACS Appl. Polym. Mater. 2020, 2, 5641–5645. [Google Scholar] [CrossRef]
- Healy, B.; Yu, T.; Alves, D.C.d.S.; Okeke, C.; Breslin, C. Cyclodextrins as Supramolecular Recognition Systems: Applications in the Fabrication of Electrochemical Sensors. Materials 2021, 14, 1668. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Kuriakose, S.; Mathew, T. Designing of a β-Cyclodextrin -based supramolecular fluorescent sensor doped with superparamagnetic α-Fe2O3 nanoparticles with improved light fastness, thermal, and photoluminescent properties. J. Appl. Polym. Sci. 2021, 138, 51004. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Votava, M.; Ravoo, B.J. Principles and applications of cyclodextrin liquid crystals. Chem. Soc. Rev. 2021, 50, 10009–10024. [Google Scholar] [CrossRef]
- Fourmentin, S.; Crini, G.; Lichtfouse, E. Cyclodextrin Fundamentals, Reactivity and Analysis; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Khan, N.A.; Durakshan, M. Cyclodextrin: An overview. Int. J. Bioassays 2013, 2, 858–865. [Google Scholar]
- Waalkens-Berendsen, D.; Prooije, A.S.-V.; Bär, A. Embryotoxicity and teratogenicity study with α-cyclodextrin in rabbits. Regul. Toxicol. Pharmacol. 2004, 39, 40–46. [Google Scholar] [CrossRef]
- Gooding, M.; Malhotra, M.; McCarthy, D.J.; Godinho, B.; Cryan, J.; Darcy, R.; O’Driscoll, C.M. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: In vitro analysis. Eur. J. Pharm. Sci. 2015, 71, 80–92. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Jia, S.; Wang, W.; Yuan, Z.; Ravoo, B.J.; Guo, D.-S. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 2018, 11, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, C.; Wu, C.; Yao, S.Q.; Wu, S. Cell-penetrating poly(disulfide)-based star polymers for simultaneous intracellular delivery of miRNAs and small molecule drugs. Polym. Chem. 2017, 8, 4043–4051. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, X.; Jin, Q.; Ji, J. ATP Suppression by pH-Activated Mitochondria-Targeted Delivery of Nitric Oxide Nanoplatform for Drug Resistance Reversal and Metastasis Inhibition. Small 2020, 16, e2001747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, W.; Li, J.; Zhang, H.; Dai, X.; Liu, Y.; Liu, Y. High-efficiency dynamic sensing of biothiols in cancer cells with a fluorescent β-cyclodextrin supramolecular assembly. Chem. Sci. 2020, 11, 4791–4800. [Google Scholar] [CrossRef] [Green Version]
- Liew, S.S.; Zeng, Z.; Cheng, P.; He, S.; Zhang, C.; Pu, K. Renal-Clearable Molecular Probe for Near-Infrared Fluorescence Imaging and Urinalysis of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 18827–18831. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Rong, R.-X.; Li, J.-M.; Chen, X.; Wang, S.-S.; Li, X.-L.; Wang, K.-R. Effective Renal Clearance and Photothermal Therapy of a Cyclodextrin-Modified Quaterrylene Derivative. ACS Appl. Bio Mater. 2020, 3, 3390–3400. [Google Scholar] [CrossRef]
- Kang, Y.; Ju, X.; Ding, L.-S.; Zhang, S.; Li, B.-J. Reactive Oxygen Species and Glutathione Dual Redox-Responsive Supramolecular Assemblies with Controllable Release Capability. ACS Appl. Mater. Interfaces 2017, 9, 4475–4484. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Zhang, Y.-M.; Li, N.; Liu, Y. Supramolecular nanoparticles based on β-CD modified hyaluronic acid for DNA encapsulation and controlled release. Chem. Commun. 2018, 54, 8713–8716. [Google Scholar] [CrossRef]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular Micelles of Amphiphilic Cyclodextrin-Core Star-Like Copolymers with Covalent pH-Responsive Linkage of Anticancer Prodrugs. Mol. Pharm. 2016, 14, 2529–2537. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, X.; Zhou, J.; Mao, Z.; Huang, X.; Wang, Z.; Hua, B.; Liu, Y.; Zhang, F.; He, Z.; et al. Supramolecular Polymer-Based Nanomedicine: High Therapeutic Performance and Negligible Long-Term Immunotoxicity. J. Am. Chem. Soc. 2018, 140, 8005–8019. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hou, B.; Xu, Z.; Saeed, M.; Sun, F.; Gao, Z.; Lai, Y.; Zhu, T.; Zhang, F.; Zhang, W.; et al. Supramolecular Prodrug Nanovectors for Active Tumor Targeting and Combination Immunotherapy of Colorectal Cancer. Adv. Sci. 2020, 7, 1903332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Hou, X.; Zhang, H.; Guo, W.; Li, H.; Jiang, L. Enantioselective Recognition in Biomimetic Single Artificial Nanochannels. J. Am. Chem. Soc. 2011, 133, 7644–7647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Xu, X.; Yu, Q.; Liu, Y.-H.; Zhang, Y.-H.; Chen, L.-X.; Liu, Y. Reversing the Cytotoxicity of Bile Acids by Supramolecular Encapsulation. J. Med. Chem. 2017, 60, 3266–3274. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.-P.; Chen, D.; Liu, C.-F.; Zhuo, L.-H.; Li, H.; Wang, C.; Bu, H.-T.; Tian, W. β-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH- and esterase- dual-responsive drug delivery. Carbohydr. Polym. 2020, 246, 116654. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2018, 17, 349–373. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2018, 96, 872–886. [Google Scholar] [CrossRef]
- Ghitman, J.; Voicu, S.I. Controlled drug delivery mediated by cyclodextrin-based supramolecular self-assembled carriers: From design to clinical performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266. [Google Scholar] [CrossRef]
- Lai, W.-F.; Rogach, A.L.; Wong, W.-T. Chemistry and engineering of cyclodextrins for molecular imaging. Chem. Soc. Rev. 2017, 46, 6379–6419. [Google Scholar] [CrossRef]
- Mehwish, N.; Dou, X.; Zhao, Y.; Feng, C.-L. Supramolecular fluorescent hydrogelators as bio-imaging probes. Mater. Horiz. 2018, 6, 14–44. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. Host-Guest Chemistry in Supramolecular Theranostics. Theranostics 2019, 9, 3041–3074. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Ding, Y.-F.; Shui, M.; Yue, L.; Gao, C.; Wyman, I.W.; Wang, R. Supramolecular biomaterials for bio-imaging and imaging-guided therapy. Eur. J. Nucl. Med. 2021, 49, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Zhang, H.-Y.; Zhang, C.; Zhang, B.; Dai, X.; Xu, X.; Liu, Y. Supramolecular Assembly Based on Sulfato-β-cyclodextrin for Hypoxia Cell Imaging. ACS Appl. Polym. Mater. 2022, 4, 2935–2940. [Google Scholar] [CrossRef]
- Jiao, J.-B.; Wang, G.-Z.; Hu, X.-L.; Zang, Y.; Maisonneuve, S.; Sedgwick, A.C.; Sessler, J.L.; Xie, J.; Li, J.; He, X.-P.; et al. Cyclodextrin-Based Peptide Self-Assemblies (Spds) That Enhance Peptide-Based Fluorescence Imaging and Antimicrobial Efficacy. J. Am. Chem. Soc. 2019, 142, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, Y.; Li, J.; He, S.; Huang, J.; Pu, K. A Renal-Clearable Macromolecular Reporter for Near-Infrared Fluorescence Imaging of Bladder Cancer. Angew. Chem. Int. Ed. 2019, 59, 4415–4420. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, Y.; Xu, L.; Wang, Y.; Geng, W.; Li, J.; Kong, X.; Zhao, X.; Zheng, Y.; Zhao, Y.; et al. Macrocyclic-Amphiphile-Based Self-Assembled Nanoparticles for Ratiometric Delivery of Therapeutic Combinations to Tumors. Adv. Mater. 2021, 33, e2007719. [Google Scholar] [CrossRef]
- Kopp, M.; Kollenda, S.; Epple, M. Nanoparticle–Protein Interactions: Therapeutic Approaches and Supramolecular Chemistry. Accounts Chem. Res. 2017, 50, 1383–1390. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Accounts Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, K.; Zheng, S.; Zhang, X.; Yan, J.; Li, W.; Zhang, A. Upper Critical Solution Temperature-Type Responsive Cyclodextrins with Characteristic Inclusion Abilities. Chem. A Eur. J. 2021, 27, 10470–10476. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; An, N.; Chen, D.; Liu, Y.; Liu, C.-P.; Yao, H.; Wang, C.; Song, X.; Tian, W. Facile construction of shape-regulated β-cyclodextrin-based supramolecular self-assemblies for drug delivery. Carbohydr. Polym. 2019, 231, 115714. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, C.; Hu, H.; Guo, X.-L.; Jiang, B.-P.; Liang, H.; Shen, X.-C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019, 10, 4699–4706. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, N.; Xiao, K.; Yu, Q.; Liu, Y. Photo-Controlled Reversible Microtubule Assembly Mediated by Paclitaxel-Modified Cyclodextrin. Angew. Chem. Int. Ed. 2018, 57, 8649–8653. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, B.; Zhang, Y.-M.; Liu, Y.-H.; Liu, Y. Actin Cytoskeleton-Disrupting and Magnetic Field-Responsive Multivalent Supramolecular Assemblies for Efficient Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 13709–13717. [Google Scholar] [CrossRef]
- Li, C.; Dou, Y.; Chen, Y.; Qi, Y.; Li, L.; Han, S.; Jin, T.; Guo, J.; Chen, J.; Zhang, J. Site-Specific MicroRNA-33 Antagonism by pH-Responsive Nanotherapies for Treatment of Atherosclerosis via Regulating Cholesterol Efflux and Adaptive Immunity. Adv. Funct. Mater. 2020, 30, 2002131. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, T.; Lin, F.-C.; Zhang, B.; Zink, J.I. Supramolecular Assemblies of Heterogeneous Mesoporous Silica Nanoparticles to Co-deliver Antimicrobial Peptides and Antibiotics for Synergistic Eradication of Pathogenic Biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Song, Y.; Zhou, N.; Wang, M.; Zhang, Z.; He, L.; Du, M. A γ-cyclodextrin-based metal–organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale 2019, 11, 20956–20967. [Google Scholar] [CrossRef]
- He, Y.; Xiong, T.; He, S.; Sun, H.; Huang, C.; Ren, X.; Wu, L.; Patterson, L.H.; Zhang, J. Pulmonary Targeting Crosslinked Cyclodextrin Metal–Organic Frameworks for Lung Cancer Therapy. Adv. Funct. Mater. 2020, 31, 2004550. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Zhang, Y.-M.; Liu, Y. Two-dimensional supramolecular assemblies based on β-cyclodextrin-grafted graphene oxide for mitochondrial dysfunction and photothermal therapy. Chem. Commun. 2019, 55, 12200–12203. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, N.; Yang, R.; Zhang, L.; Jiang, X. Folic Acid-Decorated β-Cyclodextrin-Based Poly(ε-caprolactone)-dextran Star Polymer with Disulfide Bond-Linker as Theranostic Nanoparticle for Tumor-Targeted MRI and Chemotherapy. Pharmaceutics 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Velhal, K.; Barage, S.; Roy, A.; Lakkakula, J.; Yamgar, R.; Alqahtani, M.S.; Yadav, K.K.; Ahn, Y.; Jeon, B.-H. A Promising Review on Cyclodextrin Conjugated Paclitaxel Nanoparticles for Cancer Treatment. Polymers 2022, 14, 3162. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; de la Torre, C.; Sansone, F.; Casnati, A.; Mellet, C.O.; García Fernández, J.M.; Ceña, V. Synthesis, self-assembly and anticancer drug encapsulation and delivery properties of cyclodextrin-based giant amphiphiles. Carbohydr. Polym. 2021, 252, 117135. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liang, M.; Ye, M.; Xu, J.; Liu, H.; Zhang, X.; Sun, W.; Xue, P.; Kang, Y.; Xu, Z. Reduction-triggered polycyclodextrin supramolecular nanocage induces immunogenic cell death for improved chemotherapy. Carbohydr. Polym. 2023, 301, 120365. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhang, H.; Zhang, X.; Yu, X.; Wang, Y.; Meng, Q.-F.; Yang, K.; Bai, B.; Tian, R.; Zhu, S.; et al. Supramolecular engineering of cell membrane vesicles for cancer immunotherapy. Sci. Bull. 2022, 67, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, G.; Yang, Z.; Yue, L.; Zhang, X.; Sun, C.; Wei, J.; Rao, L.; Chen, X.; Wang, R. Supramolecular Polymerization-Induced Nanoassemblies for Self-Augmented Cascade Chemotherapy and Chemodynamic Therapy of Tumor. Angew. Chem. Int. Ed. 2021, 60, 17570–17578. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qi, S.; Yu, X.; Bai, B.; Zhang, X.; Mao, Z.; Huang, F.; Yu, G. A Hybrid Supramolecular Polymeric Nanomedicine for Cascade-Amplified Synergetic Cancer Therapy. Angew. Chem. Int. Ed. 2022, 61, e202203786. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Yu, B.; Qi, Y.; Zhao, N.; Xu, F. Versatile Types of Cyclodextrin-Based Nucleic Acid Delivery Systems. Adv. Health Mater. 2020, 10, 2001183. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Y.-L.; Li, Z.; Loh, X.J. Cyclodextrin-based sustained gene release systems: A supramolecular solution towards clinical applications. Mater. Chem. Front. 2018, 3, 181–192. [Google Scholar] [CrossRef]
- Evenou, P.; Rossignol, J.; Pembouong, G.; Gothland, A.; Colesnic, D.; Barbeyron, R.; Rudiuk, S.; Marcelin, A.-G.; Ménand, M.; Baigl, D.; et al. Bridging β-Cyclodextrin Prevents Self-Inclusion, Promotes Supramolecular Polymerization, and Promotes Cooperative Interaction with Nucleic Acids. Angew. Chem. Int. Ed. 2018, 57, 7753–7758. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Li, Z.; Fan, X.; Loh, X.; Cheng, H.; Wu, Y.-L.; Li, Z. Cyclodextrin-Based Hybrid Polymeric Complex to Overcome Dual Drug Resistance Mechanisms for Cancer Therapy. Polymers 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr. Polym. 2021, 276, 118747. [Google Scholar] [CrossRef]
- Kuzuya, A.; Ohnishi, T.; Wasano, T.; Nagaoka, S.; Sumaoka, J.; Ihara, T.; Jyo, A.; Komiyama, M. Efficient Guest Inclusion by β-Cyclodextrin Attached to the Ends of DNA Oligomers upon Hybridization to Various DNA Conjugates. Bioconj. Chem. 2009, 20, 1643–1649. [Google Scholar] [CrossRef]
- Hu, L.-Z.; Wan, N.; Ma, X.-X.; Jing, Z.-W.; Zhang, Y.-X.; Li, C.; Zhou, S.-Y.; Zhang, B.-L. Enhanced gene transfection performance and biocompatibility of polyethylenimine through pseudopolyrotaxane formation with α-cyclodextrin. Nanotechnology 2017, 28, 125102. [Google Scholar] [CrossRef]
- Takashima, Y.; Oka, T.; Yoshida, S.; Yamaguchi, H.; Harada, A. Supramolecular Spherical β-Cyclodextrin32-dendrimer: Inclusion Properties and Supramolecular Structure. Chem. Lett. 2011, 40, 742–743. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, J.; Cai, L.; Ma, D.; Xue, W. Supramolecular Aggregate as a High-Efficiency Gene Carrier Mediated with Optimized Assembly Structure. ACS Appl. Mater. Interfaces 2016, 8, 29343–29355. [Google Scholar] [CrossRef]
- Liao, R.; Yi, S.; Liu, M.; Jin, W.; Yang, B. Folic-Acid-Targeted Self-Assembling Supramolecular Carrier for Gene Delivery. Chembiochem 2015, 16, 1622–1628. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Du, J.; Ren, K.; Wang, Y. Cell penetrating peptide-based polyplexes shelled with polysaccharide to improve stability and gene transfection. Nanoscale 2015, 7, 8476–8484. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, Z.; Li, J. Highly Efficient Multifunctional Supramolecular Gene Carrier System Self-Assembled from Redox-Sensitive and Zwitterionic Polymer Blocks. Adv. Funct. Mater. 2014, 24, 3874–3884. [Google Scholar] [CrossRef]
- Xie, B.; Peng, J.; Wang, S.; Zhang, X.; Nie, H. Investigation of the Sequential Actions of Doxorubicin and p53 on Tumor Cell Growth Via Branched Polyethylenimine-β-cyclodextrin Conjugates. Ann. Biomed. Eng. 2016, 44, 3372–3383. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, C.; Zhao, N.; Xu, F.-J. Redox-Responsive and Drug-Embedded Silica Nanoparticles with Unique Self-Destruction Features for Efficient Gene/Drug Codelivery. Adv. Funct. Mater. 2017, 27, 1606229. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, H.; Chang, Y.; Li, D.; Jin, H.; Zhang, M.; Wang, H.; Jiang, C.; Shen, Y.; Huang, Y. Targeting death receptors for drug-resistant cancer therapy: Codelivery of pTRAIL and monensin using dual-targeting and stimuli-responsive self-assembling nanocomposites. Biomaterials 2018, 158, 56–73. [Google Scholar] [CrossRef]
- Xiong, Q.; Bai, Y.; Shi, R.; Wang, J.; Xu, W.; Zhang, M.; Song, T. Preferentially released miR-122 from cyclodextrin-based star copolymer nanoparticle enhances hepatoma chemotherapy by apoptosis induction and cytotoxics efflux inhibition. Bioact. Mater. 2021, 6, 3744–3755. [Google Scholar] [CrossRef]
- Thelu, H.V.P.; Albert, S.K.; Golla, M.; Krishnan, N.; Ram, D.; Srinivasula, S.M.; Varghese, R. Size controllable DNA nanogels from the self-assembly of DNA nanostructures through multivalent host–guest interactions. Nanoscale 2017, 10, 222–230. [Google Scholar] [CrossRef]
- He, M.; Zhong, C.; Hu, H.; Jin, Y.; Chen, Y.; Lou, K.; Gao, F. Cyclodextrin/chitosan nanoparticles for oral ovalbumin delivery: Preparation, characterization and intestinal mucosal immunity in mice. Asian J. Pharm. Sci. 2018, 14, 193–203. [Google Scholar] [CrossRef]
- He, X.; Long, Q.; Zeng, Z.; Yang, L.; Tang, Y.; Feng, X. Simple and Efficient Targeted Intracellular Protein Delivery with Self-Assembled Nanovehicles for Effective Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1906187. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Huo, M.; Zhang, B.; Liu, Z.; Liu, Y. Folic Acid-Modified Cyclodextrin Multivalent Supramolecular Assembly for Photodynamic Therapy. Biomacromolecules 2022, 23, 3549–3559. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, X.; Zhu, H.; Wang, R.; Zhao, B.; Liu, S.; Li, Q.; Qian, W.; Feng, F.; Liu, F.; et al. Programmed cyclodextrin-based core–shell nanoparticles for cooperative TGF-β blockade to reverse immunosuppression post photodynamic therapy. Chem. Eng. J. 2023, 455, 140830. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2019, 14, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 2018, 157, 107–124. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Han, Y.; Liu, H.; Ren, F.; Zeng, J.; Sun, Q.; Li, Z.; Gao, M. Light-Enhanced O2-Evolving Nanoparticles Boost Photodynamic Therapy To Elicit Antitumor Immunity. ACS Appl. Mater. Interfaces 2019, 11, 16367–16379. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, M.; Li, B.; Xue, C.; Cai, K.; Zhao, Y.; Luo, Z. Tumor-targeted upconverting nanoplatform constructed by host-guest interaction for near-infrared-light-actuated synergistic photodynamic-/chemotherapy. Chem. Eng. J. 2020, 390, 124516. [Google Scholar] [CrossRef]
- Wu, X.; Tian, M. Fabrication of α-cyclodextrin/polypeptide micellar gold nanoshell for synergistic photothermal-chemotherapy. J. Nanopart. Res. 2018, 20, 217. [Google Scholar] [CrossRef]
- Nocito, G.; Petralia, S.; Malanga, M.; Béni, S.; Calabrese, G.; Parenti, R.; Conoci, S.; Sortino, S. Biofriendly Route to Near-Infrared-Active Gold Nanotriangles and Nanoflowers through Nitric Oxide Photorelease for Photothermal Applications. ACS Appl. Nano Mater. 2019, 2, 7916–7923. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Liu, Y.; Liu, Y. Magnetic Supramolecular Nanofibers of Gold Nanorods for Photothermal Therapy. Adv. Ther. 2018, 2, 1800137. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Chen, X.; Wang, T.; Li, L.; Su, Z.; Wang, C. Tailored Surfaces on 2D Material: UFO-Like Cyclodextrin-Pd Nanosheet/Metal Organic Framework Janus Nanoparticles for Synergistic Cancer Therapy. Adv. Funct. Mater. 2018, 28, 1803815. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Yang, H.; Ren, C.; Lv, F.; Gao, S.; Ma, H.; Jin, Y.; Ge, K.; Liu, D.; et al. Mesoporous Platinum Nanotherapeutics for Combined Chemo-photothermal Cancer Treatment. ACS Appl. Bio Mater. 2019, 2, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, M.; Pan, W.; Wang, H.; Li, N.; Tang, B. Tumor microenvironment-triggered fabrication of gold nanomachines for tumor-specific photoacoustic imaging and photothermal therapy. Chem. Sci. 2017, 8, 4896–4903. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; In, I.; Park, S.Y. pH-Responsive NIR-Absorbing Fluorescent Polydopamine with Hyaluronic Acid for Dual Targeting and Synergistic Effects of Photothermal and Chemotherapy. Biomacromolecules 2017, 18, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, J.; Tao, C.; Zhu, J.-J. Highly monodisperse beta-cyclodextrin-covellite nanoparticles for efficient photothermal and chemotherapy. Nanoscale Horiz. 2018, 3, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, L.; Xue, J.; Zhang, K.; Xu, F.-J. Degradable one-dimensional dextran-iron oxide nanohybrids for MRI-guided synergistic gene/photothermal/magnetolytic therapy. Nano Today 2021, 38, 101118. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the Tumor Microenvironment through Second-Near-Infrared-Window Photothermal Genome Editing of PD-L1 Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, e2006003. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Wang, Y.; Han, M.; Wang, C.; Yan, F. Cyclodextrin-Functionalized Gold Nanorods Loaded with Meclofenamic Acid for Improving N6-Methyladenosine-Mediated Second Near-Infrared Photothermal Immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 40612–40623. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Przybylski, C.; Bistri-Aslanoff, O.; Ménand, M.; Zhang, Y.; Sollogoub, M. Programmed Synthesis of Hepta-Differentiated β-Cyclodextrin: 1 out of 117655 Arrangements. Angew. Chem. Int. Ed. 2021, 60, 12090–12096. [Google Scholar] [CrossRef]
- Wang, B.; Zaborova, E.; Guieu, S.; Petrillo, M.; Guitet, M.; Blériot, Y.; Ménand, M.; Zhang, Y.; Sollogoub, M. Site-selective hexa-hetero-functionalization of α-cyclodextrin an archetypical C6-symmetric concave cycle. Nat. Commun. 2014, 5, 5354. [Google Scholar] [CrossRef] [Green Version]






| Guest | Other Components | Supramolecular System | Therapeutic Agents | Ref. |
|---|---|---|---|---|
| adamantane | double helix | DNA | [86] | |
| PEI | 2,4-dinitrobenzaldehyde | polyrotaxanes | DNA | [87] |
| adamantane | PAMAM dendrimer | bilayer supramolecular sphere | DNA | [88] |
| PLLD-Arg | PEG | supramolecular hydrogel | pMMP-9 | [89] |
| ferrocene | PEI, PEG | supramolecular aggregate | pMMP-9 | [90] |
| adamantane | folate acid, PEI, PEG | supramolecular self-assembly | pDNA | [91] |
| azobenzene | dextran | supramolecular self-assembly | DNA | [92] |
| adamantane | pDMAEMA, pMPC | phospholipid bilayer | DNA | [93] |
| adamantane | PEI, DOX | supramolecular self-assemble | p53, DOX | [94] |
| DTX | folate acid, DSPE, PEG | supramolecular self-assembly | DTX, siRNA | [95] |
| adamantane | MSN, PGEA, IPTS | supramolecular hybrid system | pDNA, siDNA, DOX | [96] |
| monensin | PEI, PGA | supramolecular self-assembly | DNA, TRAIL | [97] |
| DOX | PDMAEMA, PEG | supramolecular self-assembly | DOX, miR-122 | [98] |
| Guest | Other Components | Supramolecular System | PDT Type | Combined Therapy | Ref. |
|---|---|---|---|---|---|
| ferrocene | HA, poly (γ-glutamic acid), TGF-β1 inhibitor | LC@HCDFC NPs | Ce6 | immunotherapy | [104] |
| PEG | NO donor | α-CD-Ce6-NO NPs | Ce6 | chemotherapy | [105] |
| adamantane | AuNR, MSN, PEG, peptide | AuNR@MSN-CD/Ada-RLA/CS(DMA)-PEG | ICG | PTT | [106] |
| Ce6 | CS, PEG | CS–CD–Ce6 | Ce6 | immunotherapy | [107] |
| CPT | UCNPs, lactobionic acid, PEG, ROS-generating agent | UCNP@mSiO2-NBCCPT@(FITC)/β-CD-PEG-LA@DHMA | UCNPs | chemotherapy | [108] |
| Guest | Other Components | Supramolecular System | PTT Type | Combined Therapy | Ref. |
|---|---|---|---|---|---|
| pyridine-2-imine | AuNPs, DNA | Au-DNA-αCDs | AuNPs | photoacoustic imaging | [114] |
| azobenzene | transferrin, polylysine, PEG | TPM-Azo⊂GOCD | GO | mitochondrial physical disruption | [72] |
| paclitaxel | HA, TPP | FNPs-pDA@HA-TPP-CD-PTX | Polydopamine | chemotherapy | [115] |
| adamantane | RGD, DOX | CuS-DOX | CuS | chemotherapy | [116] |
| adamantane | Dextran, Fe3O4, PGEA, p53 | Fe3O4@Dex-PGEA | Fe3O4 | magnetolytic therapy | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Ye, B.; Yu, G.; Huang, F.; Mao, Z.; Ding, Y.; Wang, W. Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review. Molecules 2023, 28, 3441. https://doi.org/10.3390/molecules28083441
Hu W, Ye B, Yu G, Huang F, Mao Z, Ding Y, Wang W. Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review. Molecules. 2023; 28(8):3441. https://doi.org/10.3390/molecules28083441
Chicago/Turabian StyleHu, Wenting, Binglin Ye, Guocan Yu, Feihe Huang, Zhengwei Mao, Yuan Ding, and Weilin Wang. 2023. "Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review" Molecules 28, no. 8: 3441. https://doi.org/10.3390/molecules28083441
APA StyleHu, W., Ye, B., Yu, G., Huang, F., Mao, Z., Ding, Y., & Wang, W. (2023). Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review. Molecules, 28(8), 3441. https://doi.org/10.3390/molecules28083441