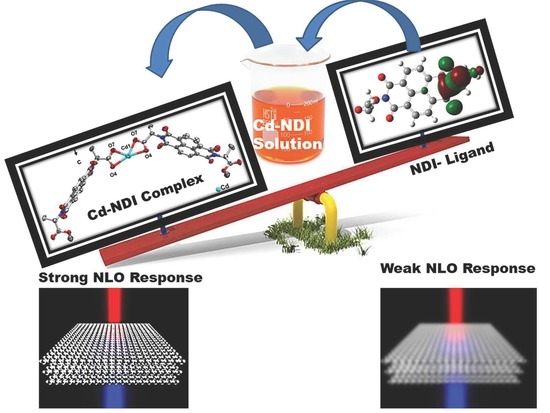
The Electronic Properties of Cadmium Naphthalene Diimide Coordination Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure of Complex
2.2. Hirschfield Surface Analysis
2.3. Optimized Geometries
2.4. Photophysical Properties
2.5. Frontier Molecular Orbitals (FMOs)
2.6. Density of States (DOS)
2.7. Dipole Moment (µ)
2.8. Transition Density Matrix (TDM)
2.9. Molecular Electrostatic Potential Map (MEP) Analysis
2.10. Electron Density Distribution Matrix (EDDM)
2.11. Non-Covalent Interaction (NCI) Analysis
2.12. Infrared (IR) Analysis

2.13. Cyclic Voltammetry (CV)
2.13.1. Electrical Conductivity (σ)
2.13.2. Electronic Transition of NDI and PDI Ligands
2.14. NLO Properties
2.14.1. Linear Isotropic and Anisotropic Polarizabilities
2.14.2. The First Hyperpolarizability
2.14.3. The Second Hyperpolarizability
3. Experimental
3.1. Materials and Instrumentation
3.2. Single-Crystal Analysis
Crystal Structure Refinement Details of Cd-NDI Complex
3.3. Computational Details
3.4. Syntheses of Ligand
3.5. Complex Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahsin, A.; Ayub, K. Theoretical investigation of superalkali clusters M2OCN and M2NCO (where M= Li, Na, K) as excess electron system with significant static and dynamic nonlinear optical response. Optik 2021, 227, 166037. [Google Scholar] [CrossRef]
- Rahaman, S.J.; Samanta, A.; Mir, M.H.; Dutta, B.J.E.M. Manufacturing, Metal-Organic Frameworks (MOFs): A Promising Candidate for Stimuli-Responsive Drug Delivery. ES Mater. Manuf. 2022, 19, 792. [Google Scholar]
- Hales, J.M.; Cozzuol, M.; Screen, T.E.; Anderson, H.L.; Perry, J.W. Metalloporphyrin polymer with temporally agile, broadband nonlinear absorption for optical limiting in the near infrared. Opt. Express 2009, 17, 18478–18488. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Baudequin, C.; Plé, N. Luminescent materials incorporating pyrazine or quinoxaline moieties. Dye. Pigment. 2013, 98, 575–600. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Karakas, A.; Elmali, A.; Unver, H. Linear optical transmission measurements and computational study of linear polarizabilities, first hyperpolarizabilities of a dinuclear iron (III) complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Baloach, R.; Ayub, K.; Mahmood, T.; Asif, A.; Tabassum, S.; Gilani, M.A. A new strategy of bi-alkali metal doping to design boron phosphide nanocages of high nonlinear optical response with better thermodynamic stability. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3062–3076. [Google Scholar] [CrossRef]
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S.J.C.R. Borates: A rich source for optical materials. Chem. Rev. 2020, 121, 1130–1202. [Google Scholar] [CrossRef]
- Ishaq, M.; Shehzad, R.A.; Yaseen, M.; Iqbal, S.; Ayub, K.; Iqbal, J. DFT study of superhalogen-doped borophene with enhanced nonlinear optical properties. J. Mol. Model. 2021, 27, 188. [Google Scholar] [CrossRef]
- Ahmed, F.; Dutta, B.; Mir, M.H.J.D.T. Electrically conductive 1D coordination polymers: Design strategies and controlling factors. Dalton Trans. 2021, 50, 29–38. [Google Scholar] [CrossRef]
- Muhammad, S.; Janjua, M.R.S.A.; Su, Z. Investigation of dibenzoboroles having π-electrons: Toward a new type of two-dimensional NLO molecular switch? J. Phys. Chem. C 2009, 113, 12551–12557. [Google Scholar] [CrossRef]
- Shehzad, R.A.; Muhammad, S.; Iqbal, J.; Al-Sehemi, A.G.; Yaseen, M.; Aloui, Z.; Khalid, M. Exploring the optoelectronic and third-order nonlinear optical susceptibility of cross-shaped molecules: Insights from molecule to material level. J. Mol. Model. 2021, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Muhammad, S.; Khera, R.A.; Shehzad, R.A.; Ayub, K.; Iqbal, J. DFT study of superhalogen (AlF4) doped boron nitride for tuning their nonlinear optical properties. Optik 2021, 231, 166464. [Google Scholar] [CrossRef]
- Ji, M.; Hu, C.-L.; Li, Y.; Li, B.; Mao, J.-G.J.C.o.M. Ag[SC(NH2)2]2F: A Laser Damage-Tolerant Infrared Nonlinear Optical Material. Chem. Mater. 2022, 34, 9753–9759. [Google Scholar] [CrossRef]
- Xiao, D.; Bulat, F.A.; Yang, W.; Beratan, D.N. A donor—nanotube paradigm for nonlinear optical materials. Nano Lett. 2008, 8, 2814–2818. [Google Scholar] [CrossRef]
- Mutailipu, M.; Li, F.; Jin, C.; Yang, Z.; Poeppelmeier, K.R.; Pan, S.J.A.C. Strong nonlinearity induced by coaxial alignment of polar chain and dense [BO3] units in CaZn2 (BO3)2. Angew. Chem. 2022, 134, e202202096. [Google Scholar] [CrossRef]
- Zahid, S.; Rasool, A.; Ayub, A.R.; Ayub, K.; Iqbal, J.; Al-Buriahi, M.; Alwadai, N.; Somaily, H. Silver cluster doped graphyne (GY) with outstanding non-linear optical properties. RSC Adv. 2022, 12, 5466–5482. [Google Scholar] [CrossRef]
- Lee, M.J.; Piao, M.; Jeong, M.-Y.; Lee, S.H.; Kang, K.M.; Jeon, S.-J.; Lim, T.G.; Cho, B.R. Novel azo octupoles with large first hyperpolarizabilities. J. Mater. Chem. 2003, 13, 1030–1037. [Google Scholar]
- Zhang, M.; Wang, X.; Sun, H.; Yu, J.; Wang, N.; Long, Y.; Huang, C. Preparation of room-temperature ferromagnetic semiconductor based on graphdiyne-transition metal hybrid. 2d Mater. 2018, 5, 035039. [Google Scholar] [CrossRef]
- Bhaskar, A.; Guda, R.; Haley, M.M.; Goodson, T., III. Building symmetric two-dimensional two-photon materials. J. Am. Chem. Soc. 2006, 128, 13972–13973. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Graphyne and graphdiyne: Promising materials for nanoelectronics and energy storage applications. J. Phys. Chem. C 2012, 116, 5951–5956. [Google Scholar] [CrossRef]
- Qiu, Y.-Q.; Li, Z.; Ma, N.-N.; Sun, S.-L.; Zhang, M.-Y.; Liu, P.-J. Third-order nonlinear optical properties of molecules containing aromatic diimides: Effects of the aromatic core size and a redox-switchable modification. J. Mol. Graph. Model. 2013, 41, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, L. Recent advances in naphthalenediimide-based metal-organic frameworks: Structures and applications. Coord. Chem. Rev. 2021, 430, 213665. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, K.; Gu, L.-l.; Li, H. The study of perylene diimide–amino acid derivatives for the fluorescence detection of anions. RSC Adv. 2017, 7, 42685–42689. [Google Scholar] [CrossRef]
- Chen, Q.; Xian, S.; Dong, X.; Liu, Y.; Wang, H.; Olson, D.H.; Williams, L.J.; Han, Y.; Bu, X.H.; Li, J. High-Efficiency Separation of n-Hexane by a Dynamic Metal-Organic Framework with Reduced Energy Consumption. Angew. Chem. 2021, 133, 10687–10691. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Wei, Y.; Li, W.; Zhu, Y. π–π Interaction between self-assembled perylene diimide and 3D graphene for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 240, 225–233. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Lu, F.; Liu, J.; Chen, X.; Shao, G. Boosting aqueous Zn2+ storage in 1, 4, 5, 8-naphthalenetetracarboxylic dianhydride through nitrogen substitution. ChemElectroChem 2019, 6, 3644–3647. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Yamani, Z.H.; Jamil, S.; Mahmood, A.; Ahmad, I.; Haroon, M.; Tahir, M.H.; Yang, Z.; Pan, S. First principle study of electronic and non-linear optical (NLO) properties of triphenylamine dyes: Interactive design computation of new NLO compounds. Aust. J. Chem. 2015, 69, 467–472. [Google Scholar] [CrossRef]
- Li, X.; Li, S. Investigations of electronic and nonlinear optical properties of single alkali metal adsorbed graphene, graphyne and graphdiyne systems by first-principles calculations. J. Mater. Chem. C 2019, 7, 1630–1640. [Google Scholar] [CrossRef]
- Zahid, S.; Rasool, A.; Ans, M.; Yaseen, M.; Iqbal, J. Quantum chemical approach of donor− π–acceptor based arylborane–arylamine macrocycles with outstanding photovoltaic properties toward high-performance organic solar cells. Energy Fuels 2021, 35, 15018–15032. [Google Scholar] [CrossRef]
- Rasool, A.; Zahid, S.; Shehzad, R.A.; Akhter, M.S.; Iqbal, J. Designing of benzodithiophene (BDT) based non-fullerene small molecules with favorable optoelectronic properties for proficient organic solar cells. Comput. Theor. Chem. 2021, 1203, 113359. [Google Scholar] [CrossRef]
- Khan, A.U.; Khera, R.A.; Anjum, N.; Shehzad, R.A.; Iqbal, S.; Ayub, K.; Iqbal, J. DFT study of superhalogen and superalkali doped graphitic carbon nitride and its non-linear optical properties. RSC Adv. 2021, 11, 7779–7789. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Kosar, N.; Arshad, M.N.; Gilani, M.A.; Ayub, K.; Mahmood, T. Design of novel superalkali doped silicon carbide nanocages with giant nonlinear optical response. Opt. Laser Technol. 2020, 122, 105855. [Google Scholar] [CrossRef]
- Zahid, S.; Rasool, A.; Shehzad, R.A.; Bhatti, I.A.; Iqbal, J. Tuning the optoelectronic properties of triphenylamine (TPA) based small molecules by modifying central core for photovoltaic applications. J. Mol. Model. 2021, 27, 237. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, H. Fine-Tuning Aromatic Stacking and Single-Crystal Photoluminescence Through Coordination Chemistry. Eur. J. Org. Chem. 2019, 2019, 1778–1783. [Google Scholar] [CrossRef]
- Drissi, M.; Benhalima, N.; Megrouss, Y.; Rachida, R.; Chouaih, A.; Hamzaoui, F. Theoretical and experimental electrostatic potential around the m-nitrophenol molecule. Molecules 2015, 20, 4042–4054. [Google Scholar] [CrossRef]
- Kiran, R.; Khera, R.A.; Khan, A.U.; Ayoub, A.; Iqbal, N.; Ayub, K.; Iqbal, J. Study of nonlinear optical properties of superhalogen and superalkali doped phosphorene. J. Mol. Struct. 2021, 1236, 130348. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Abdullah, H.Y. Vinyl chloride adsorption onto the surface of pristine, Al-, and Ga-doped boron nitride nanotube: A DFT study. Solid State Commun. 2021, 337, 114440. [Google Scholar] [CrossRef]
- Doust Mohammadi, M.; Abdullah, H.Y. The adsorption of chlorofluoromethane on pristine, and Al-and Ga-doped boron nitride nanosheets: A DFT, NBO, and QTAIM study. J. Mol. Model. 2020, 26, 287. [Google Scholar] [CrossRef]
- Lee, K.A.; Lozan, V.; Langford, S.J.; Kersting, B.J.D.T. Ternary complexes composed of naphthalene diimides and binucleating metallocavitands: Preparation, characterisation and structure of [(Ni2L) 2 (NDI)][BPh4] 2. Dalton Trans. 2009, 36, 7481–7485. [Google Scholar] [CrossRef]
- Li, X.; Lu, J. Giant enhancement of electronic polarizability and the first hyperpolarizability of fluoride-decorated graphene versus graphyne and graphdiyne: Insights from ab initio calculations. Phys. Chem. Chem. Phys. 2019, 21, 13165–13175. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.-M.; Xu, K.-W.; Ji, V. A first-principles study on gas sensing properties of graphene and Pd-doped graphene. Appl. Surf. Sci. 2015, 343, 121–127. [Google Scholar] [CrossRef]
- Khalid, M.; Khan, M.U.; Ahmed, S.; Shafiq, Z.; Alam, M.M.; Imran, M.; Braga, A.A.C.; Akram, M.S. Exploration of promising optical and electronic properties of (non-polymer) small donor molecules for organic solar cells. Sci. Rep. 2021, 11, 21540. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Iqbal, J.; Khalid, M.; Hussain, R.; Braga, A.A.C.; Hussain, M.; Muhammad, S. Designing triazatruxene-based donor materials with promising photovoltaic parameters for organic solar cells. RSC Adv. 2019, 9, 26402–26418. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Price Mortimer, A.J.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef]
- Al-Yasari, A.; Van Steerteghem, N.; El Moll, H.; Clays, K.; Fielden, J. Donor–acceptor organo-imido polyoxometalates: High transparency, high activity redox-active NLO chromophores. Dalton Trans. 2016, 45, 2818–2822. [Google Scholar] [CrossRef]
- Khan, M.U.; Khalid, M.; Ibrahim, M.; Braga, A.A.C.; Safdar, M.; Al-Saadi, A.A.; Janjua, M.R.S.A. First theoretical framework of triphenylamine–dicyanovinylene-based nonlinear optical dyes: Structural modification of π-linkers. J. Phys. Chem. C 2018, 122, 4009–4018. [Google Scholar] [CrossRef]
- Khan, M.U.; Ibrahim, M.; Khalid, M.; Qureshi, M.S.; Gulzar, T.; Zia, K.M.; Al-Saadi, A.A.; Janjua, M.R.S.A. First theoretical probe for efficient enhancement of nonlinear optical properties of quinacridone based compounds through various modifications. Chem. Phys. Lett. 2019, 715, 222–230. [Google Scholar] [CrossRef]
- Khan, M.U.; Ibrahim, M.; Khalid, M.; Braga, A.A.C.; Ahmed, S.; Sultan, A. Prediction of second-order nonlinear optical properties of D–π–A compounds containing novel fluorene derivatives: A promising route to giant hyperpolarizabilities. J. Clust. Sci. 2019, 30, 415–430. [Google Scholar] [CrossRef]
- Doan, D.-Q.; Fang, T.-H.; Tran, A.-S.; Chen, T.-H. High deformation capacity and dynamic shear band propagation of imprinted amorphous Cu50Zr50/crystalline Cu multilayered nanofilms. J. Phys. Chem. Solids 2020, 138, 109291. [Google Scholar] [CrossRef]
- Nazir, R.; Yaqoob, J.; Khan, M.U.; Gilani, M.A.; Hussain, R.; Alvi, M.U.; Rashid, M.; Assiri, M.A.; Imran, M. Computational study of 2N-atom functionalized corannulene by alkali metals doping: Towards the development of highly efficient nonlinear optical materials. Phys. B Condens. Matter 2022, 640, 414041. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Z.; Chen, C.; Lee, M.-H. Mechanism of linear and nonlinear optical effects of KDP and urea crystals. J. Chem. Phys. 2003, 118, 2349–2356. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Shehzad, R.A.; Iqbal, J.; Ayub, K.; Nawaz, F.; Muhammad, S.; Ayub, A.R.; Iqbal, S. Enhanced linear and nonlinear optical response of superhalogen (Al7) doped graphitic carbon nitride (g-C3N4). Optik 2021, 226, 165923. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.-L.; Wang, M.-S. The organic co-crystals formed using naphthalenediimide-based triangular macrocycles and coronene: Intermolecular charge transfers and nonlinear optical properties. Phys. Chem. Chem. Phys. 2022, 24, 29747–29756. [Google Scholar] [CrossRef]
- Lucenti, E.; Cariati, E.; Dragonetti, C.; Manassero, L.; Tessore, F. Effect of the Coordination to the “Os3 (CO) 11” Cluster Core on the Quadratic Hyperpolarizability of trans-4-(4 ‘-X-styryl) pyridines (X= NMe2, t-Bu, CF3) and trans, trans-4-(4 ‘-NMe2-phenyl-1, 3-butadienyl) pyridine. Organometallics 2004, 23, 687–692. [Google Scholar] [CrossRef]
- Tessore, F.; Di Carlo, G.; Forni, A.; Righetto, S.; Limosani, F.; Biroli, A.O. Second Order Nonlinear Optical Properties of 4-Styrylpyridines Axially Coordinated to A4 ZnII Porphyrins: A Comparative Experimental and Theoretical Investigation. Inorganics 2020, 8, 45. [Google Scholar] [CrossRef]
- Prasad, G.K.; Prashanth, S.; Srivastava, S.; Rao, G.N.; Babu, D.R. Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes. Open Chem. 2017, 15, 283–292. [Google Scholar] [CrossRef]
- Trujillo, A.; Fuentealba, M.; Carrillo, D.; Manzur, C.; Ledoux-Rak, I.; Hamon, J.-R.; Saillard, J.-Y. Synthesis, spectral, structural, second-order nonlinear optical properties and theoretical studies on new organometallic donor− acceptor substituted nickel (II) and Copper (II) unsymmetrical Schiff-base complexes. Inorg. Chem. 2010, 49, 2750–2764. [Google Scholar] [CrossRef]



















| Identification Code | Cd-NDI Complex |
|---|---|
| Empirical formula | C40H28.65CdN4O17.32 |
| Formula weight | 954.83 |
| T (K) | 296.15 |
| Crystal system | Orthorhombic |
| Space group | P21212 |
| a (Å) | 24.891(2) |
| b (Å) | 7.3061(7) |
| c (Å) | 11.7772(11) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 2141.7(3) |
| Z | 2 |
| ρcald (g/cm3) | 1.481 |
| µ (Mo Kα) (mm−1) | 0.588 |
| F(000) | 966.0 |
| Crystal size/mm3 | 0.25 × 0.23 × 0.2 |
| Radiation | Mo Kα (λ = 0.71073) |
| 2Θ range for data collection/° | 3.458 to 50.76 |
| Index ranges | −29 ≤ h ≤ 30, −8 ≤ k ≤ 8, −14 ≤ l ≤ 14 |
| Reflections collected | 21,339 |
| Unique data (Rint) | 3935 (0.0251) |
| Rsigma | 0.0206 |
| Data/restraints/parameters | 3935/6/290 |
| GOF | 1.061 |
| R1 /wR2 [I > 2(Iσ)] | 0.0442/0.1273 |
| R1 /wR2 [all data] | 0.0464/0.1298 |
| maximum/minimum (e/Å−3) | 2.19/−0.41 |
| Flack parameter | −0.003(8) |
| D | H | A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| O1 | H1 | O4 1 | 0.82 | 1.80 | 2.584(6) | 158.2 1 |
| Excited State | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| E(eV) | 1.196 eV | 1.340 eV | 1.386 eV | 1.428 eV | 1.526 eV |
| λmax (nm) | 322 | 319 | 317 | 315 | 305 |
| D(Å) | 12.651 | 7.416 | 5.090 | 20.768 | 10.744 |
| F | 0.0394 | 0.5093 | 0.4130 | 0.0075 | 0.0006 |
| S | 0.17161 | 0.39821 | 0.41036 | 0.01619 | 0.31276 |
| Transition mode | CT | CT | CT | CT | CT |
| Excited State | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| E(eV) | 3.84 | 3.882 eV | 3.906 eV | 3.934 eV | 3.946 eV |
| λmax (nm) | 1036.6 | 925.2 | 894.3 | 868.2 | 853.8 |
| D(Å) | 4.251 | 1.111 | 1.119 | 6.158 | 1.686 |
| F | 0.0004 | 0.0007 | 0.0015 | 0.0045 | 0.0001 |
| S | 0.19131 | 0.53443 | 0.53190 | 0.25832 | 0.39650 |
| Transition mode | CT | CT | CT | CT | CT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, W.; Khan, M.A.; Li, Z.; Iqbal, M.J.; Ilyas, M.; Li, H. The Electronic Properties of Cadmium Naphthalene Diimide Coordination Complex. Molecules 2023, 28, 3709. https://doi.org/10.3390/molecules28093709
Hussain W, Khan MA, Li Z, Iqbal MJ, Ilyas M, Li H. The Electronic Properties of Cadmium Naphthalene Diimide Coordination Complex. Molecules. 2023; 28(9):3709. https://doi.org/10.3390/molecules28093709
Chicago/Turabian StyleHussain, Wajid, Maroof Ahmad Khan, Zhongkui Li, Muhammad Javed Iqbal, Mubashar Ilyas, and Hui Li. 2023. "The Electronic Properties of Cadmium Naphthalene Diimide Coordination Complex" Molecules 28, no. 9: 3709. https://doi.org/10.3390/molecules28093709
APA StyleHussain, W., Khan, M. A., Li, Z., Iqbal, M. J., Ilyas, M., & Li, H. (2023). The Electronic Properties of Cadmium Naphthalene Diimide Coordination Complex. Molecules, 28(9), 3709. https://doi.org/10.3390/molecules28093709