1. Introduction
Histamine is a biogenic amine synthesized through enzymatic decarboxylation of histidine and plays various physiological and metabolic roles [
1]. It is produced and stored in basophils and mast cells, and is also found in gastric cells that trigger gastric acid release and histaminergic neurons. In addition, during inflammation initiated by an allergen, histamine is released by mast cells, inducing allergic or inflammatory reactions and modulating innate and adaptative immune responses [
2].
Histamine is also found in low concentrations in food and beverages, particularly in some fruits and vegetables, seafood, fish, matured cheese, and other fermented food [
3]. In rotten food, however, toxic levels of histamine (>500 mg/kg) can lead to food poisoning, often a direct result of bacterial growth in the food matrix.
Histamine toxicity, also called “scombroid fish poisoning”, is linked to microbial protease and decarboxylase activity in spoiled or rotten fish and other high protein/high histidine food matrices.
Furthermore, histamine buildup indicates product freshness and proper storage, especially in fish products. Therefore, histamine detection and quantification are crucial for food safety and quality assurance [
3]. Additionally, early histamine detection is a critical process for niche applications such as low histamine food products for the histamine-hypersensitive population, which—as for other food intolerances—is steadily increasing, currently affecting approximately 1% of the population [
4].
Current histamine analysis methods are based on chromatographic analytics for biogenic amines, such as thin-layer chromatography and high-performance liquid chromatography (TLC, HPLC), which have been well-summarized [
5]. Histamine can be separated through HPLC using a C18 reversed-phase column and an acetonitrile−water gradient, which is also applied for the detection of biogenic amines, usually employing pre- or postcolumn derivatization using o-phtalaldehyde (OPA) or dansyl chloride [
6] under alkaline and reducing conditions for colorimetric determination or using o-phtaldehyde (OPT) for fluorogenic detection. HPLC separation coupled with high-resolution mass spectrometry allows for decreasing the detection limit for histamine, reaching 5 mg/kg in fish meat [
5] and 0.07 ng/mL in baby food [
6]. Despite achieving impressive detection methods, analytical methods for quantifying histamine (and other biogenic amines) require sample concentration, purification, advanced instrumentation, and specialized operators.
Alternatively, enzyme-based detection of histamine is a promising alternative to analytic methods. Two main types of enzymatic approaches have been described: electro-enzymatic and colorimetric. Histamine oxidases (HOD, EC 1.4.3.6) catalyze the oxidative deamination of primary amines (e.g., histamine), releasing ammonia and hydrogen peroxide. For example, the development of enzyme-coated electrodes allowed the electrochemical detection of various biogenic amines, including histamine, allowing the detection of 3 µM histamine [
7].
In enzyme-based chromogenic assays, histamine is degraded by Histamine Dehydrogenase (HDH, EC 1.4.9.1) in the presence of an electron carrier (1-methoxy-5-methylphenazinium methylsulfate, 1-PMS), producing reduced 1-methoxy PMS, transferring electrons to a chromogenic tetrazolium salt (WST-8, or MTT), and the product can be measured at 460 nm (
Figure 1A). These colorimetric assays present a valuable opportunity for developing affordable histamine detection kits that could be routinely used for food quality assurance and control.
HDHs have activity against cadaverine, putrescine, tyramine, tryptamine, spermine, and spermidine (
Figure 1B).
Rhizobium sp. HDH (Rsp HDH) showed agmatine oxidation at a rate of 10% that of histamine [
8] and 1,3-Diaminopropane at a 13% rate of histamine [
9]. In contrast, HDH from
Nocardioides simplex (HDH-N) converted putrescine at 30% the rate exhibited with histamine [
10]. Available HDHs also suffer from substrate inhibition, especially from the primary substrate, histamine. The latter affects enzyme performance in complex samples, hindering the accuracy and specificity of enzyme-based histamine detection and quantification methods [
11,
12]. Thus, HDHs with high histamine specificity and reduced substrate inhibition are needed to develop specific histamine colorimetric methods for the food industry.
Enzyme limitations such as substrate specificity profiles and inhibition can be improved using protein engineering. Protein engineering strategies aim to obtain enzymes with higher performance than the starting variant [
13]. Directed evolution is the most used strategy in enzyme engineering [
14,
15]. Improved variants can be generated and identified using iterative cycles of gene diversity generation and high throughput screening. In addition, specific enzyme residues and residues based on in silico, structural, and computational chemistry approaches can be targeted through rational and semi-rational protein engineering approaches [
13,
14,
15,
16].
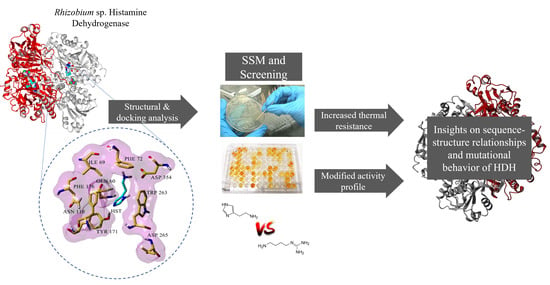
In this work, we studied the effect of site saturation mutagenesis in selected amino acid residues near the proposed histamine-binding site in Rhizobium sp. Histamine Dehydrogenase (Rsp HDH) to identify novel structure–function relationships contributing to decreasing its activity against other biogenic amines and reducing substrate inhibition. For this, we performed in silico structural analysis of Rsp HDH, selected amino acid positions for site saturation mutagenesis (SSM), screened for histamine and agmatine activity using a microtiter plate format colorimetric assay, selected for variants with improved histamine/agmatine activity ratios, and maintained thermal resistance. Then, selected variants were characterized, and their structure–function changes were studied in silico.
2. Results and Discussion
The results are presented as the structural analysis of Rsp HDH to select amino acid sites for SSM, construction of the SSM libraries, library screening, amino acid substitution combination, variant characterization regarding substrate activity and thermal resistance compared against WT Rsp HDH, and structure–function analysis of the selected variants.
The crystal structures for
Nocardioides simplex HDH (PDB ID: 3K30) [
17] and
Rhizobium sp. HDH (PDB ID: 6DE6) are available (
Figure 2A). They are structurally similar [
10,
17], with each chain containing a 6-S-Cys-FMN and [4Fe-4S] as redox-active cofactors and a non-covalently bound ADP molecule [
18]. In addition,
Nocardioides simplex HDH has proposed histamine binding sites (Gln65, Ile74, Phe77, Glu79, Asn115, Tyr181, Trp267, Asp358), which are potential targets for mutagenesis studies [
10,
17].
The structure of Rsp HDH (693 aa, Uniprot Q60I59) was used as a starting point for this study since it has a more histamine-specific substrate spectrum and high thermal resistance [
8,
9], making it an attractive starting point for protein engineering. However, its structure was released in 2019, with a yet-to-be-released accompanying research publication providing more detailed information. For analysis, the structure was energy minimized using YASARA Structure [
19,
20], and the resulting structure was aligned with the structure of the Histamine Dehydrogenase of
Nocardiopsis simplex (PDB ID: 3K30) (
Figure 2B), where residues involved in catalysis and substrate binding have been described [
15]. This structural alignment allowed us to identify the corresponding residues in Rsp HDH potentially involved in catalysis and substrate [
17,
21]. Thus, residues Gln60, Glu74, Phe72, Tyr171, His174, Arg225, Trp263, and Asp265 in Rsp HDH formed the active site. Furthermore, the catalytic triad in Rsp HDH comprises Tyr171, His174, and Asp265. To identify amino acid positions probably having direct contact with the substrate, mechanism-based [
22,
23] molecular docking experiments were performed using the minimized structure of Rsp HDH.
Docking results show the best catalytically competent docking pose, with a calculated binding energy of −4.37 kcal/mol (
Figure S1). The resulting distance between the N atom of histamine and the N5 of FMN was 2.655 Å (
Table S1). Furthermore, the imidazole ring of histamine was stabilized via a pi-stacking interaction with Trp263, His174, and Tyr171 (
Figure 2C). The imidazole ring of histamine interacted with F176 and Tyr171, whereas the ethylamine group could form hydrophobic interactions with the residues Phe72, Glu74, Cys30, and Trp263. These observations suggest that hydrophobic interactions modulate substrate binding in Rsp HDH.
Additionally, the residues aligning in active site cavities and tunnels were identified using Caver web tool 1.0 [
24]. In total, 66 lining residues with Pocket relevance scores of 100%, volume 4255 A
3, and draggability: 0.63 values were identified (
Figure S2 and Table S2). Furthermore, residues Gln60, Ile69, Phe72, Glu74, Asn110, Phe131, Tyr171, Ala173, His174, Gly175, Phe176, Thr262, Trp263, Asp354, Met355, and Leu567 were identified by the Caver web tool as the residues constitute the tunnel one, which seems important for the substrate transport to the active site of the
Rsp HDH (
Figure S3 and Table S3). Finally, integrating the structural alignment, docking results, distance from histamine, and cavity analysis, residues Gln60, Ile69, Phe72, Asn110, Tyr171, Phe176, Trp263, Asp265, and Asp354 were selected as the target for site saturation mutagenesis to explore their effect in HDH activity.
The SSM libraries were generated using Quick change PCR [
25], introducing NNK degenerate codons through specifically designed primers for each library (
Table S4), and the libraries were transformed into
E. coli BL21 Gold (DE3) cells for activity screening (~90 colonies per library), approximately 3× the necessary amount of colonies needed to cover the codon diversity [
16]. The correct construction for each library was confirmed using DNA sequencing (through diversity introduction in the specific codon for the PCR product). Overall, ~1000 colonies were picked from transformation agar plates directly to stock microtiter plates (MTPs).
The screening assay was based on a colorimetric assay where the biogenic amine substrate was degraded by HDH, producing reduced 1-methoxy PMS and transferring electrons to a chromogenic tetrazolium salt (WST-8), resulting in an increase in absorbance at 460 nm [
8], adapted to the 96-well MTP format (
Figure 1A). The assay was validated by growing and inducing 96
E. coli clones producing WT Rsp HDH in an MTP and obtaining a standard deviation for the measured activity value below 15%, which is acceptable for mutant library screening [
16].
For SSM library screening, two activity measurements were performed per clone, one using histamine and another using agmatine as substrate (
Figure 1B), both at 320 µM. HDH from
N. simplex has shown activity against histamine, agmatine, and putrescine, with
Km values of 31, 37, and 1280 mM, respectively [
18]. On the other hand, HDH for
Rhizobium sp., despite having high substrate specificity [
9], showed oxidation of agmatine and 1,3-diaminopropane at 10% and 13% the rate of histamine oxidation, respectively. Thus, increased substrate specificity is desired for histamine detection and quantification applications [
9,
18].
Screening for histamine activity allowed us to keep track of the main activity of the variants, whereas measuring agmatine activity allowed for calculating the histamine/agmatine activity ratio, describing changes in substrate specificity independently from the total activity measured for each clone.
After screening all nine SSM libraries, we observed differences between the histamine activity profile across the variant population.
Figure 3 shows screening plates where the measured histamine activity of the clones has been plotted in decreasing order. For libraries Tyr171, Phe176, Trp263, Asp265, and Asp354, the vast majority of the clones in the plate had no activity, and all identified active clones had no amino acid substitutions. Evolutionary conservation analysis of each residue was performed using Consurf [
26] and UET servers [
27] (
Figures S4 and S5). Consurf analysis revealed that Tyr171, Phe176, and Trp263 scored nine, seven, and nine, respectively, showing that they are highly conserved residues (
Table S5).
This amino acid substitution sensitivity suggests these positions are critical for enzyme function. The structural equivalents of Tyr171 and Asp265 in
N. simplex HDH (Tyr176 and Asp270) are described as part of the catalytic triad [
17]. On the other hand, positions Trp 263 and Phe 176 did not align structurally with relevant residues in
N. simplex HDH. However, position Trp 267 of the latter was reported to be part of an “aromatic bowl” for substrate binding [
17]; interestingly, in the work of Tsutsumi et al. [
21], three positions in
N. simplex HDH close to the structural equivalents of Phe176 and Trp263 in Rsp HDH, namely Tyr181, Gly269, and Asp270 (numbered Tyr 180, Gly268, and Asp269 in the cited work), were subjected to site-directed mutagenesis to Phe180, Asp269, and Cys269, reporting changes in the redox potential of the enzyme, leading to changes in the kinetic parameters and substrate inhibition profiles.
An intermediate situation was observed for positions Gln60 and Ile69, where active clones were identified but with much lower histamine dehydrogenase activity than WT Rsp HDH, suggesting these amino acid positions are somewhat optimized for histamine already. Gln60 and Ile69 were identified as less conserved, with Consurf scores of seven and six, respectively (
Figures S4 and S5 and Table S5).
On the other hand, for libraries Phe72 and Asn110, a wide range of activity values were found, suggesting these positions are less sensitive for amino acid substitutions in the case of histamine as substrate and probably not yet optimized for the screened criteria. Additionally, these two residues were identified as variable residues by Consurf analysis (
Figures S4 and S5 and Table S5).
Since variants from mutagenesis libraries at positions 72 and 110 were selected for increased histamine/agmatine activity ratio, a double SSM library (Phe72/Asn110) was constructed for Rsp HDH. In addition, the increased theoretical diversity from introducing two simultaneous NNK codons in the gene (32 × 32 codon combinations) meant that more than 3000 variants needed to be screened to reach at least 95% diversity coverage.
We screened the double SSM library for increased histamine/agmatine activity ratio using 320 µM for histamine and 1000 µM agmatine. Additionally, we included another screening measurement using 1000 µM histamine to assess substrate inhibition, which is reported for Histamine Dehydrogenases, specifically for
N. simplex and Rsp HDH [
9,
18,
21]. Finally, since combining two amino acid substitutions could reduce enzyme stability or thermal resistance, we included an additional measurement by incubating the enzyme-containing lysate at 60 °C for 20 min, followed by a centrifugation step to ensure the selected variants would have a comparable thermal resistance to WT Rsp HDH, which might be necessary for further technical application of the enzyme.
The screening revealed several variants with increased histamine/agmatine ratios; however, those with higher activity ratios only showed amino acid substitutions at positions 72 or 110 (
Figure 4A). Substitutions appearing more often for position 72 among rescreened variants were Trp, Tyr, and His, and Leu, Val, Thr, and Ser were found less frequently. On the other hand, for position 110, Val, Trp, Gly, and Thr were found more often, whereas Leu, Pro, Arg, and His were also found. Interestingly, Trp, with only one codon in thirty-two available, was seen several times in rescreened variants at either position 72 or 110, strongly suggesting that this bulky hydrophobic residue plays a role in the substrate binding or the reaction mechanism; however, no variant with Trp simultaneously at positions 72 and 110 was identified, whereas Tyr72 was found in combination with other amino acid substitutions for variants selected for decreased substrate inhibition.
When histamine/agmatine (substrate specificity) and histamine 1000 µM/histamine 320 µM (substrate inhibition) activity ratios were considered, it could be proposed that positions 72 and 110 affect both substrate specificity and substrate inhibition.
From the identified variants, two were selected for further evaluation, Phe72Thr and Asn110Val, which also showed good histamine/agmatine ratio performance. Interestingly, for both screening parameters, no variants with double substitutions showed higher performance than the single-substituted Phe72Thr and Asn110Val variants, probably related to low compatibility for amino acid combinations at positions 72 and 110 or a selection bias resulting from applying multiple selection criteria during screening, such as thermal resistance.
Variants Phe72Thr and Asn110Val were purified using immobilized metal affinity chromatography (IMAC) and compared regarding kinetic parameters using histamine and agmatine as substrates (
Figure 4B,C). Enzymatic activity for histamine and agmatine was measured via the initial reaction rate (linear range) in microtiter plates as described in Materials and Methods, at substrate concentrations from 0 to 2000 µM. As previously described for this enzyme, a clear substrate inhibition curve for histamine was observed for all variants [
9]. Therefore, the substrate saturation was adjusted to the substrate inhibition equation (
V =
Vmax×[S]/(
Km + X×(1 + [S]/
Ki))) (
Figure 4B). On the other hand, for agmatine, a Michaelis–Menten fitting (
V = (
Vmax×[S]/(
Km + [S])) was used to describe the saturation curves (
Figure 4C).
Vmax,
Km, and
Ki values obtained for these fittings were used as reference values to compare the catalytic behavior of both enzyme variants against WT Rsp HDH (
Table 1).
For histamine,
Vmax values increased for the variants compared with those for WT Rsp HDH, especially for variant Phe72Thr (398 compared with 216 µmol WST-8 formazan per mg enzyme per min). On the other hand,
Km values increased for both variants, usually observed when screening was performed at substrate concentrations higher than
Km, and variants showing higher activity were selected [
28]. Thus, the
Vmax/
Km values for the variants were reduced for histamine compared with those for WT Rsp HDH. Finally, observed
Ki values for the variants decreased compared with those for WT Rsp HDH, meaning that the inhibition effect of the substrate should be observed at lower concentrations if the
Vmax and
Km values were the same as WT Rsp HDH. Variant Asn110Val showed a similar
Vmax value to variant WT Rsp HDH. Still, the smaller
Ki value meant substrate inhibition was observed at lower concentrations for Asn110Val, thus hindering the observed activity at histamine concentrations higher than 250 µM.
For agmatine, an overall decrease in the specific activity was observed for the HDH variants. The Vmax value decreased for Asn110Val and Phe72Thr compared with that for WT Rsp HDH. On the other hand, the Km value did not change dramatically and was reduced for variant Phe72Thr to 112.9 µM, whereas for variant Asn110Val, it was increased to 170.1 µM. Consequently, the Vmax/Km values were reduced compared with those for WT Rsp HDH.
Overall, variant Phe72Thr showed an increased activity against histamine and a decreased activity against agmatine when compared with WT Rsp HDH; however, activity at lower substrate concentrations was decreased for the variant, explained by the increased Km value of 194.6 µM, compared with the original 38.24 µM of the WT Rsp HDH. Therefore, selecting enzyme variants with higher Vmax and higher Km is common when screening using substrate concentrations above Km. On the other hand, when screening using substrate concentrations under Km, there is a risk of selecting variants with lower activity at higher concentrations, and in this case, also with lower Ki values, meaning a higher substrate inhibition at lower histamine concentrations.
The activity profile of purified WT Rsp HDH and variant Phe72Thr against histamine and other biogenic amine was measured at 320 µM for each substrate (
Figure 5A), evidencing a desired decrease in calculated specific activity of variant Phe72Thr for agmatine and 1,3-diaminopropane, both substrates reported as the main secondary substrates for
Rhizobium sp. HDH [
9,
11]. Interestingly, the selected variant showed a sharper decrease in activity for 1,3-diaminopropane, a substrate not used in the screening process since agmatine was reported as a common alternative substrate for both HDHs [
9,
17]. However, this decrease in activity suggests that activity against agmatine and 1,3-diaminopropane could co-evolve in screening events. Additionally, this indicates that substrate recognition for the amine moiety is probably similar to that for agmatine and 1,3-diaminopropane. Therefore, this work shows the observed correlation for these molecules as interferents for HDH and in the observed activity profile of the variant Phe72Thr.
Additionally, activity measurements using histamine saturation curves were performed for WT Rsp HDH and variant Phe72Thr in the presence of a biogenic amine mixture (“BA mix”) containing agmatine, tyramine, cadaverine, putrescine, and 1,3-diaminopropane (200 µM each, 1000 µM total, (
Figure 5B), or in the presence of 1000 µM agmatine, (
Figure 5C)) to observe the interfering effect of these molecules on HDH activity, simulating complex samples for histamine quantification. In both cases, a decrease in activity was observed for the HDH variant at the lowest substrate range; however, the shape of the enzyme activity curves was very different for Wt Rs HDH and Phe72Thr. The former, in the presence of both 1000 µM BA mix and 1000 µM agmatine, showed maximum activity at around 100 µM histamine, maintaining and decreasing the observed activity at higher histamine concentration, similar to the substrate inhibition curve observed in
Figure 5B. On the other hand, the activity of variant Phe72Thr increased with higher histamine concentration, both in the presence of 1000 µM BA mix and 1000 µM agmatine, up to 2000 µM histamine.
For Phe72Thr, instead of substrate inhibition, a substrate saturation behavior was observed when other biogenic amines were present in the reaction, contrary to the behavior observed for the pure histamine activity curve. This change in activity versus substrate concentration response could be useful for histamine quantification applications, since it could be modeled to relate enzyme activity and histamine concentration for a wider substrate range (up to 2000 µM histamine) than WT Rs HDH, which, despite having a higher sensitivity, peaks at 100 µM histamine. Furthermore, this change in activity profile also suggests that position Phe72 could be involved not only in substrate specificity but also in substrate inhibition in Rsp HDH, as suggested by other authors for
N. simplex HDH. There could be a close relationship between catalysis rate and substrate inhibition, where a decrease in substrate inhibition is linked to an increased K
m for histamine [
21], which was also observed in this work for the Phe72Thr variant, compared with WT Rsp HDH.
Finally, a thermal resistance profile was performed to assess the residual activity of WT Rsp HDH and the variant Phe72Thr after incubation at different temperatures (
Figure 6A). Variant Phe72Thr was expected to be at least as thermally resistant as WT Rsp HDH since a heat shock step was included during the screening process for the simultaneous SSM libraries. Variant Phe72Thr showed an increase in thermal resistance, with no activity loss after 20 min incubation at 65 °C, whereas WT Rsp HDH kept ~70% of its initial activity after the same process. After incubation at 75 °C, variant Phe72Thr maintained ~40% of the initial activity; in contrast, WT Rsp HDH showed ~20% of the initial activity.
Figure 6B shows that after heat shock treatment, recombinant WT Rsp HDH and the Phe72Thr variant remain soluble (lanes 3 and 4).
In contrast, most other proteins present in
E. coli lysate were not found in the supernatant after centrifugation. This increase in thermal resistance may be due to activity/stability compensation where the observed reduced affinity for the substrate could increase the interaction with water, thus increasing the overall stability of the enzyme [
29,
30]. By improving its initial thermal resistance, variant Phe72Thr is an interesting candidate for further enzyme processing, such as quick purification via thermal unfolding or immobilization in solid supports for quantitative color-based (strip type) histamine detection applications.
Computational analysis was conducted to elucidate structure–function relationships on generated Rsp HDH variants regarding potential interaction with histamine, agmatine, and 1,3-diaminopropane. First, the Phe72Thr substitution was introduced using FoldX Suite [
31,
32], and energy was minimized. Then, independently, WT Rsp HDH and the Phe72Thr variant were used as receptors for molecular docking of histamine (
Figure 7A), agmatine (
Figure 7B), and 1,3-diaminopropane (
Figure 7C). As stated above, according to the catalytic mechanism proposed for HDHs [
22,
23], the distance between the N5 atom of FMN and the N atom of the amino group in the substrate was used to identify catalytically competent binding modes. The closest distance for histamine in WT Rsp HDH was calculated to be 2.655 Å, with −4.37 kcal/mol binding energy.
In contrast, the Phe72Thr variant had a 2.679 Å distance and −4.39 kcal/mol binding energy, slightly lower than the value for WT Rsp HDH. Furthermore, the two other tested substrates had a longer distance than histamine, which correlates well with experimental results in which the WT enzyme had a higher affinity for histamine. In addition, the Phe72Thr variant showed a longer distance for agmatine and 1,3-diaminopropane compared with that for WT Rps HDH, which could explain the lower activity of the Phe72Thr variant towards these substrates compared with the original enzyme.