Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae)
Abstract
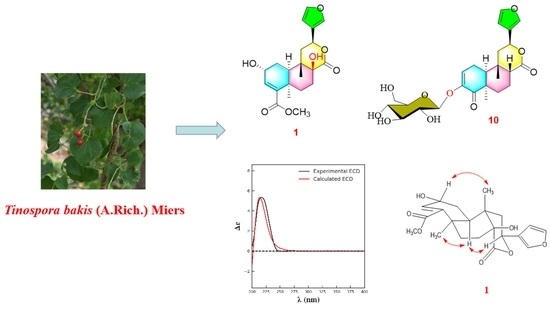
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experiment Procedures
3.2. Plant Material
3.3. Extraction and Isolation
- Tinobakisin (1): white needle-like crystals; −18.18 (c , MeOH); UV λmax 244 nm; IR (KBr) νmax 3310, 2943, 2832, 1738, 1589, 1427, 1280, 1073, 1020, 697, 668 cm−1; CD [nm (mdeg)]: 217 (5.34); 1H-NMR and 13C-NMR data, see Table 1; EI-MS [M]+, m/z 390.3; HR-EI-MS [M]+ m/z 390.1687 (calculated for 390.1679, C21H26O7).
- Tinobakiside (10): amorphous, colorless semisolid; −110.16 (c , MeOH); UV λmax 248 nm; IR (KBr) νmax 3405, 2918, 1724, 1676, 1506, 1463, 1441, 1389, 1252, 1075, 1021, 602 cm−1; CD [nm (mdeg)]: 216 (5.23); 1H-NMR and 13C-NMR data, see Table 1; FAB-MS [M + H]+ m/z 493.1; HR-FAB-MS [M + H]+ m/z 493.2061 (calculated for 493.2074, C25H33O6).
3.4. Acid Hydrolysis
3.5. ECD Calculation
3.6. Anti-Glycation Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of Diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural Products in Diabetes Research: Quantitative Literature Analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Summary of Revisions: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Gong, W.C. Natural Products Used for Diabetes. J. Am. Pharm. Assoc. 2002, 42, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ríos1, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Chi, S.; She, G.; Han, D.; Wang, W.; Liu, Z.; Liu, B. Genus Tinospora: Ethnopharmacology, Phytochemistry, and Pharmacology. Evid.-Based Complement. Altern. Med. 2016, 2016, 9232593. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Abbas Bukhari, S.N. Tinospora Species: An Overview of Their Modulating Effects on the Immune System. J. Ethnopharmacol. 2017, 207, 67–85. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhuri, P.K. Chemistry and Pharmacology of Tinospora cordifolia. Nat. Prod. Commun. 2017, 12, 299–308. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, G.J.; Liu, Y.F.; Wang, H.S.; Liang, D. Clerodane Diterpenoid Glucosides from the Stems of Tinospora sinensis. J. Nat. Prod. 2017, 80, 975–982. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, S.; Ma, F.; Li, Q.; Asada, Y.; Koike, K. Clerodane Diterpenoids from Tinospora sagittata (Oliv) Gagnep. Planta Med. 2012, 78, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Li, W.J.; Jiao, L.X.; Guo, J.M.; Tian, K.; Yang, C.T.; Huang, X.Z. New Clerodane Diterpenes from Tinospora sagittata var. yunnanensis. Planta Med. 2014, 80, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Ismail, M.; Shaari, K.; Abbaskhan, A.; Sattar, S.A.; Lajis, N.H.; Atta-Ur-Rahman. Cis-Clerodane-Type Furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 2010, 73, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Cheng, C.M.; Dai, Y.; Fu, G.M.; Guo, J.M.; Yin, Y.; Liang, H. A Novel 18-Norclerodane Diterpenoid from the Roots of Tinospora sagittata var. yunnanensis. Molecules 2010, 15, 8360–8365. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Wazir, V.; Tyagi, A.; Kapil, R.S. Clerodane Diterpenoids from Tinospora cordifolia. Phytochemistry 1995, 38, 659–661. [Google Scholar] [CrossRef]
- Khan, M.A.; Gray, A.I.; Waterman, P.G. Tinosporaside, an 18-Norclerodane Glucoside from Tinospora cordifolia. Phytochemistry 1989, 28, 273–275. [Google Scholar] [CrossRef]
- Ibrahim Abdelwahab, S.; Syaed Koko, W.; Mohamed Elhassan Taha, M.; Mohan, S.; Achoui, M.; Ameen Abdulla, M.; Rais Mustafa, M.; Ahmad, S.; Ibrahim Noordin, M.; Lip Yong, C.; et al. In Vitro and in Vivo Anti-Inflammatory Activities of Columbin through the Inhibition of Cycloxygenase-2 and Nitric Oxide but Not the Suppression of NF-ΚB Translocation. Eur. J. Pharmacol. 2012, 678, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Alamin, M.A.; Yagi, A.I.; Yagi, S.M. Evaluation of Antidiabetic Activity of Plants Used in Western Sudan. Asian Pac. J. Trop. Biomed. 2015, 5, 395–402. [Google Scholar] [CrossRef]
- Zafinindra, L.R.; Diatta, W.; Dieye, A.M.; Nongonierma, R.; Faye, B.; Bassene, E. Antipyretic Effect of Aqueous Extract and Alcaloid of Tinospora bakis (Miers) in Rabbits. Dakar Med. 2003, 48, 29–33. [Google Scholar]
- Koko, W.S.; Mesaik, M.A.; Yousaf, S.; Galal, M.; Choudhary, M.I. In Vitro Immunomodulating Properties of Selected Sudanese Medicinal Plants. J. Ethnopharmacol. 2008, 118, 26–34. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Ibrahim, N. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.R.; El-Nashar, H.A.S.; Fayez, S.; Obaid, W.A.; Eldahshan, O.A. Ameliorative Effect of Oregano (Origanum vulgare) versus Silymarin in Experimentally Induced Hepatic Encephalopathy. Sci. Rep. 2022, 12, 17854. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Yan, G.; Sun, H.; Yang, L.; Kong, L.; Sun, Y.; Han, Y.; Zhao, Q.; Kang, S.; Wang, X. Version Updates of Strategies for Drug Discovery Based on Effective Constituents of Traditional Chinese Medicine. Acupunct. Herb. Med. 2023, 3, 158–179. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane Diterpenes: Sources, Structures, and Biological Activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Mizuno, K.; Tajima, R.; Takeya, K. Furanoditerpene Glucosides from Fibraurea tinctoria. Phytochemistry 1986, 25, 905–908. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, P.L.; Zhou, M.X.; Shen, T.; Zou, Y.X.; Lou, H.X.; Wang, X.N. New Nor-Clerodane-Type Furanoditerpenoids from the Rhizomes of Tinospora capillipes. Phytochem. Lett. 2016, 15, 225–229. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, H.; Hu, S.; Xu, H.; Yang, B.; Yang, Q.; Xue, Y.; Cheng, L.; Jiang, J.; Zhang, J.; et al. Clerodane-Type Diterpenoids from Tuberous Roots of Tinospora sagittata (Oliv.) Gagnep. Fitoterapia 2016, 110, 59–65. [Google Scholar] [CrossRef]
- Roslund, M.U.; Tähtinen, P.; Niemitz, M.; Sjöholm, R. Complete Assignments of the 1H and 13C Chemical Shifts and JH,H Coupling Constants in NMR Spectra of d-Glucopyranose and All d-Glucopyranosyl-d-Glucopyranosides. Carbohydr. Res. 2008, 343, 101–112. [Google Scholar] [CrossRef]
- Yonemitsu, M.; Fukuda, N.; Kimura, T.; Komori, T. Studies on the Constituents of Jateorhiza palmata Miers (Colombo Root), II. Separation and Structure of Six New Furanoid Diterpene Glucosides: Palmatoside B, C, D, E, F, and G. Liebigs Ann. Chem. 1987, 1987, 193–197. [Google Scholar] [CrossRef]
- Yonemitsu, M.; Fukuda, N.; Kimura, T.; Komori, T. Studies on the Constituents of Jateorhiza palmata Miers (Colombo Root), I Separation and Structure of a New Furanoid Diterpene Glucoside (Palmatoside A). Liebigs Ann. Chem. 1986, 1986, 1327–1333. [Google Scholar] [CrossRef]
- Itokawa, H.; Mizuno, K.; Ichihara, Y.; Takeya, K. Isolation and 13C-NMR Studies on Three New Furanoditerpenyl Glucosides from Jateorrhiza columba. Planta Med. 1987, 53, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Goddard, R.; Akhtar, F. The Structure of Jateorin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 1217–1220. [Google Scholar] [CrossRef]
- Swaminathan, K.; Sinha, U.C.; Bhatt, R.K.; Sabata, B.K.; Tavale, S.S. Structure of Tinosporide, a Diterpenoid Furanolactone from Tinospora cordifolia Miers. Acta Crystallogr. C 1989, 45 Pt 1, 134–136. [Google Scholar] [CrossRef]
- Zakaria, M.; Saito, I.; Yao, X.-K.; Wang, R.-J.; Matsuura, T. Furanoditerpenes of Fibraurea chloroleuca. Planta Med. 1989, 55, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Hanuman, J.B.; Bhatt, R.K.; Sabata, B. A Clerodane Furano-Diterpene from Tinospora cordifolia. J. Nat. Prod. 1988, 51, 197–201. [Google Scholar] [CrossRef]
- Hanuman, J.B.; Bhatt, R.K.; Sabata, B.K. A Diterpenoid Furanolactone from Tinospora cordifolia. Phytochemistry 1986, 25, 1677–1680. [Google Scholar] [CrossRef]
- Shi, L.M.; Li, R.Q.; Liu, W.H. Two New Furanoid Diterpenoids from Tinospora cagittata. Helv. Chim. Acta 2008, 91, 978–982. [Google Scholar] [CrossRef]
- Rishikesan, R.; Raju, R.; Manikandaselvi, S.; Thinagarbabu, R.; Sivasubramanian, A. Isolation and Characterisation of Clerodane Diterpenoids from the Traditional Medicinal Plant-Tinospora glabra (Burm. f.) Merrill. Pharm. Lett. 2016, 8, 280–287. [Google Scholar]
- Oguakwa, J.U.; Galeffi, C.; Nicoletti, M. Research on African Medicinal Plants. XI. 8-Hydroxycolumbin, a New Furanoid Diterpene from Chasmanthera Dependens. Planta Med. 1986, 52, 198–199. [Google Scholar] [CrossRef]
- Chin, Y.W.; Lim, S.W.; Kim, S.H.; Shin, D.Y.; Suh, Y.G.; Kim, Y.B.; Kim, Y.C.; Kim, J. Hepatoprotective Pyrrole Derivatives of Lycium chinense Fruits. Bioorg. Med. Chem. Lett. 2003, 13, 79–81. [Google Scholar] [CrossRef]
- Leena, P.N.; Aleykutty, N.A. Isolation and Spectral Identification of Quercetin Fron the Alcoholic Root Extract of Clerodendrum paniculatum. Int. J. Pharma Sci. Res. 2016, 7, 47–50. [Google Scholar]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of Stigmasterol and β-Sitosterol from the Dichloromethane Extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Faizi, S.; Ali, M.; Saleem, R.; Irfanullah; Bibi, S. Spectral Assignments and Reference Data: Complete 1H and 13C NMR Assignments of Stigma-5-En-3-O-β-Glucoside and Its Acetyl Derivative. Magn. Reson. Chem. 2001, 39, 399–405. [Google Scholar] [CrossRef]
- Otun, K.O.; Olatunji, G.A.; Ajiboye, A.T.A.; Badeggi, U.M. Isolation and Characterization of the Chemical Constituents of the Stem Bark of Parinari polyandra Benth. Int. Res. J. Pure Appl. Chem. 2014, 4, 710–717. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, Y.Q.; Li, Y.C.; Lan, Q.; Liao, H.B.; Wang, H.S.; Liang, D. Flavonol Glycosides and Phenylpropanoid Glycosides with Inhibitory Effects on Microglial Nitric Oxide Production from Neoshirakia japonica. Fitoterapia 2021, 151, 104877. [Google Scholar] [CrossRef]
- Dai, L.; He, S.; Zhang, B.; Wang, H.; Wang, Y.; Liang, D. Foegraecumoside O and P, a Pair of Triterpenoid saponins with a 4/5/6 Fused Tricyclic Oleanane Carbon Skeleton from Lysimachia foenum-graecum Hance. Molecules 2023, 28, 5061. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Lan, Q.; Su, B.-J.; Wang, Y.; Liang, D. Three New Phenolic Glycosides and a New Lignan Glycoside from Gaultheria leucocarpa var. yunnanensis. Fitoterapia 2024, 172, 105740. [Google Scholar] [CrossRef]
- Ali, R.; Salawu, K.M.; Aamer, M.; Jahan, H.; Tufail, P.; Irshad, R.; Khan, F.A.; Sener, B.; Choudhary, M.I.; Wang, Y. A New Sesquiterpene, Prosoterpene, from Prosopis africana (Guill. & Perr.) Taub. Nat. Prod. Res. 2023, 37, 3220–3227. [Google Scholar] [CrossRef]





| No. | 1 a | 10 b | ||
|---|---|---|---|---|
| δH | δC, Multipilicity | δH | δC, Multipilicity | |
| 1 | 2.27 overlapped 2.04 overlapped | 30.1, CH2 | 2.90 ddd (20.0, 6.5, 2.5) 2.54 dd (20.0, 6.5) | 22.5, CH2 |
| 2 | 4.46 m | 65.1, CH | 6.30 dd (6.5, 2.5) | 124.3, CH |
| 3 | 6.57 d (3.2) | 140.4, CH | - | 149.2, C |
| 4 | - | 141.1, C | - | 200.5, C |
| 5 | - | 36.0, C | - | 46.8, C |
| 6 | 2.03 overlapped 1.75 m | 30.6, CH2 | 2.28 overlapped 1.06 td (14.0, 4.0) | 30.9, CH2 |
| 7 | 2.10 overlapped 1.64 ddd (14.0, 10.0, 2.8) | 39.6, CH2 | 2.16 dq (14.0, 4.0) 1.72 tt (14.0, 4.0) | 20.3, CH2 |
| 8 | - | 90.4, C | 2.47 br t (4.0) | 50.1, CH |
| 9 | - | 50.0, C | - | 37.6, C |
| 10 | 1.92 d (5.6) | 47.0, CH | 2.29 overlapped | 45.3, CH |
| 11 | 2.27 overlapped 2.05 overlapped | 47.0, CH2 | 2.37 dd (15.0, 4.0) 1.80 dd (15.0, 12.5) | 41.3, CH2 |
| 12 | 5.15 dd (11.2, 4.8) | 74.3, CH | 5.57 dd (12.5, 4.0) | 72.3, CH |
| 13 | - | 127.6, C | - | 126.8, C |
| 14 | 6.73 d (1.2) | 110.9, CH | 6.54 d (2.0) | 109.6, CH |
| 15 | 7.39 t (1.2) | 144.1, CH | 7.49 t (2.0) | 145.0, CH |
| 16 | 7.54 brs | 142.0, CH | 7.59 br s | 141.4, CH |
| 17 | - | 178.3, C | - | 174.8, C |
| 18 | - | 169.5 | ||
| 19 | 1.44 s | 30.6, CH3 | 1.28 s | 29.0, CH3 |
| 20 | 1.05 s | 22.2, CH3 | 0.97 s | 26.6, CH3 |
| 1′ | - | 4.62 d (7.0) | 102.3, CH | |
| 2′ | - | 3.38 overlapped | 74.6, CH | |
| 3′ | - | 3.32 overlapped | 78.3, CH | |
| 4′ | - | 3.34 overlapped | 71.3, CH | |
| 5′ | - | 3.39 overlapped | 77.6, CH | |
| 6′ | - | 3.84 dd (12.0, 1.8) 3.65 dd (12.0, 5.2) | 62.5, CH2 | |
| OCH3 | 3.73 s | 52.1, CH3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabbashi, A.S.; Sattar, M.A.; Aamer, M.; Siddiqui, N.N.; Kamran, M.; Fayaz, A.; Jahan, H.; Khan, F.-A.; Wang, Y. Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae). Molecules 2024, 29, 154. https://doi.org/10.3390/molecules29010154
Kabbashi AS, Sattar MA, Aamer M, Siddiqui NN, Kamran M, Fayaz A, Jahan H, Khan F-A, Wang Y. Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae). Molecules. 2024; 29(1):154. https://doi.org/10.3390/molecules29010154
Chicago/Turabian StyleKabbashi, Ahmed Saeed, Maazah Abdul Sattar, Muhammad Aamer, Nimra Naz Siddiqui, Muhammad Kamran, Aneela Fayaz, Humera Jahan, Farooq-Ahmad Khan, and Yan Wang. 2024. "Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae)" Molecules 29, no. 1: 154. https://doi.org/10.3390/molecules29010154
APA StyleKabbashi, A. S., Sattar, M. A., Aamer, M., Siddiqui, N. N., Kamran, M., Fayaz, A., Jahan, H., Khan, F. -A., & Wang, Y. (2024). Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae). Molecules, 29(1), 154. https://doi.org/10.3390/molecules29010154