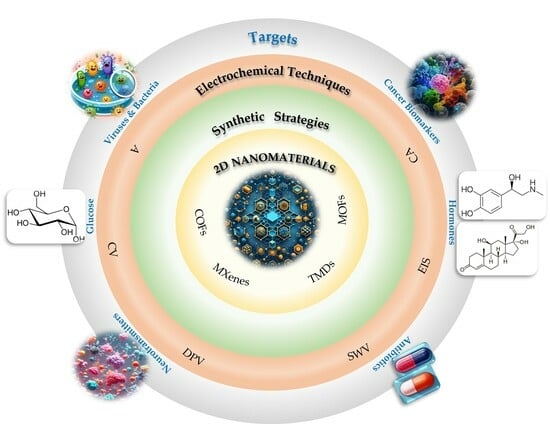
Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications
Abstract
:1. Introduction
2. Two-Dimensional Nanomaterials
2.1. MXenes
2.2. TMDs
2.3. MOFs
2.4. COFs
3. Applications of 2D Nanomaterials to Electrochemical (Bio)Sensors for Healthcare and Biomedical Field
3.1. Glucose
3.2. Neurotransmitters
3.2.1. Dopamine
3.2.2. Serotonin
3.3. Hormones
3.4. Pathogens
3.4.1. Bacteria
3.4.2. Viruses
3.5. Cancer Biomarkers
3.6. Antibiotics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.; Radoi, A.; Rashid, S.; Hayat, A.; Vasilescu, A.; Silvana Andreescu, S. Two-Dimensional Nanostructures for Electrochemical Biosensor. Sensors 2021, 21, 3369. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Brazaca, L.C.; dos Santos, L.P.; de Oliveira, P.R.; Rocha, D.P.; Stefano, J.S.; Kalinke, C.; Abarza Munoz, R.A.; Alves Bonacin, J.; Janegitz, B.C.; Carrilho, E. Biosensing strategies for the electrochemical detection of viruses and viral diseases—A review. Anal. Chim. Acta 2021, 1159, 338384. [Google Scholar] [CrossRef] [PubMed]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860. [Google Scholar] [CrossRef]
- Varghese, S.S.; Varghese, S.H.; Swaminathan, S.; Singh, K.K.; Mittal, V. Two-Dimensional Materials for Sensing: Graphene and Beyond. Electronics 2015, 4, 651–687. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Nikoleli, G.-P.; Pingarrón, J.M.; Nikolelis, D.P. Hybrid 2D-nanomaterials-based electrochemical immunosensing strategies for clinical biomarkers determination. Biosens. Bioelectron. 2017, 89, 269–279. [Google Scholar] [CrossRef]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-Dimensional Materials in Biosensing and Healthcare: From In Vitro Diagnostics to Optogenetics and Beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-Dimensional Nanomaterials beyond Graphene for Biomedical Applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Y.; Gu, Z.; Zhao, Y. Research trends in biomedical applications of two-dimensional nanomaterials over the last decade—A bibliometric analysis. Adv. Drug Deliv. Rev. 2022, 188, 114420. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024. [Google Scholar] [CrossRef]
- Arulraj, A.; Mangalaraja, R.V.; Khalid, M. MXene: Pioneering 2D Materials. In Fundamental Aspects and Perspectives of Mxenes, 1st ed.; Khalid, M., Nirmala Grace, A., Arulraj, A., Numan, A., Eds.; Springer Nature: Berlin, Germany, 2022; pp. 17–37. [Google Scholar]
- Koyappayil, A.; Ganpat Chavan, S.; Roh, Y.-G.; Lee, M.-H. Advances of MXenes; Perspectives on Biomedical Research. Biosensors 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, R.; Thadathil, D.A.; Varghese, A. New horizons in surface topography modulation of MXenes for electrochemical sensing toward potential biomarkers of chronic disorders. Crit. Rev. Solid State Mater. Sci. 2023, 48, 580–622. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D.; Li, M.; Zhou, C.; Yu, D.; Lin, Y. Recent advances in flexible and wearable chemo and bio-sensors based on two-dimensional transition metal carbides and nitrides MXenes. J. Mater. Chem. B 2022, 10, 2113. [Google Scholar] [CrossRef]
- Ab Latif, F.E.; Numan, A.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Manaf, N.A.; Walvekar, R. Evolution of MXene and its 2D heterostructure in electrochemical sensor applications. Coord. Chem. Rev. 2022, 471, 214755. [Google Scholar] [CrossRef]
- Sajid, M. MXenes: Are they emerging materials for analytical chemistry applications?—A review. Anal. Chim. Acta 2021, 1143, 267–280. [Google Scholar] [CrossRef]
- Tao, W.; Kong, N.; Ji, X.; Zhang, Y.; Sharma, A.; Ouyang, J.; Qi, B.; Wang, J.; Xie, N.; Kang, C.; et al. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019, 48, 2891. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Bi, S. Two-Dimensional Quantum Dot-Based Electrochemical Biosensors. Biosensors 2022, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Dourandish, Z.; Garkani Nejad, F.; Beitollahi, H.; Jahani, P.M.; Di Bartolomeo, A. Transition metal dichalcogenides: Synthesis and use in the development of electrochemical sensors and biosensors. Biosens. Bioelectron. 2022, 216, 114674. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, D.; Yue, X.; Hong, R.; Yang, W.; Liu, C.; Xu, H.; Lu, J.; Dong, L.; Wang, G.; et al. A Review of Transition MetalDichalcogenides-Based Biosensors. Front. Bioeng. Biotechnol. 2022, 10, 941135. [Google Scholar]
- Zhao, Y.; Wang, S.-B.; Chen, A.-Z.; Kankala, R.K. Nanoarchitectured assembly and surface of two-dimensional (2D) transition metal dichalcogenides (TMDCs) for cancer therapy. Coord. Chem. Rev. 2022, 472, 214765. [Google Scholar] [CrossRef]
- Lam, C.Y.C.; Zhang, Q.; Yin, B.; Huang, Y.; Wang, H.; Yang, M.; Wong, S.H.D. Recent Advances in Two-Dimensional Transition Metal Dichalcogenide Nanocomposites Biosensors for Virus Detection before and during COVID-19 Outbreak. J. Compos. Sci. 2021, 5, 190. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Hossein Abdollahi, S.; Safavi, A. Sugar-Based Natural Deep Eutectic Mixtures as Green Intercalating Solvents for High-Yield Preparation of Stable MoS2 Nanosheets: Application to Electrocatalysis of Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 5896–5906. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Hossein Abdollahi, S.; Omidvar, A.; Mohajeri, A.; Safavi, A. Aqueous solutions of carbohydrates are new choices of green solvents for highly efficient exfoliation of two-dimensional nanomaterials. J. Mol. Liq. 2020, 309, 113087. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Rocha, D.P.; Matias, T.A.; Juliao, M.S.S.; Munoz, R.A.A.; Angnes, L. Recent trends and perspectives in electrochemical sensors based on MOF-derived materials. J. Mater. Chem. C 2021, 9, 8718. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef]
- Haider, J.; Shahzadi, A.; Akbar, M.U.; Hafeez, I.; Shahzadi, I.; Khalid, A.; Ashfaq, A.; Ahmad, S.O.A.; Dilpazir, S.; Imran, M.; et al. A review of synthesis, fabrication, and emerging biomedical applications of metal-organic frameworks. Biomater. Adv. 2022, 140, 213049. [Google Scholar] [CrossRef] [PubMed]
- Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Garkani Nejad, F.; Sheikhshoaie, I.; Di Bartolomeo, A. A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors 2022, 22, 2238. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Periñán, E.; Martínez-Fernández, M.; Segura, J.L.; Lorenzo, E. Electrochemical (Bio)Sensors Based on Covalent Organic Frameworks (COFs). Sensors 2022, 22, 4758. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wen, W.; Tian, Z.; Zhang, X.; Wang, S. Covalent organic framework: A state-of-the-art review of electrochemical sensing applications. Talanta 2023, 260, 124613. [Google Scholar]
- Lu, Z.; Wang, Y.; Li, G. Covalent Organic Frameworks-Based Electrochemical Sensors for Food Safety Analysis. Biosensors 2023, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; O’Kelley, S. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Rodriguez-Mendez, M.L. Electrochemical Sensor for Food Monitoring Using Metal-Organic Framework Materials. Chemosensors 2023, 11, 357. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Chocholova, E.; Jane, E.; Vikartovska, A.; Kasak, P.; Tkac, J. Electrochemical Impedance Spectroscopy Based Biosensors: Mechanistic Principles, Analytical Examples and Challenges towards Commercialization for Assays of Protein Cancer Biomarkers. ChemElectroChem 2019, 6, 989–1003. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem. Soc. Rev. 2021, 50, 619. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Lib, H.-K.; He, H. Recent advances in metal/covalent organic framework-based electrochemical aptasensors for biosensing applications. Dalton Trans. 2021, 50, 14091. [Google Scholar] [CrossRef] [PubMed]
- Uçar, A.; Aydogdu Tıg, G.; Er, E. Recent advances in two dimensional nanomaterial-based electrochemical (bio)sensing platforms for trace-level detection of amino acids and pharmaceuticals. Trends Anal. Chem. 2023, 162, 117027. [Google Scholar] [CrossRef]
- Ganesan, S.; Ramajayam, K.; Kokulnathan, T.; Palaniappan, A. Recent Advances in Two-Dimensional MXene-Based Electrochemical Biosensors for Sweat Analysis. Molecules 2023, 28, 4617. [Google Scholar] [CrossRef]
- Lee, C.W.; Suh, J.M.; Jang, H.W. Chemical Sensors Based on Two-Dimensional (2D) Materials for Selective Detection of Ions and Molecules in Liquid. Front. Chem. 2019, 7, 708. [Google Scholar] [CrossRef]
- Balkourani, G.; Damartzis, T.; Brouzgou, A.; Tsiakaras, P. Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review. Sensors 2022, 22, 355. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors 2022, 12, 467. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Sohail, M.; Liaqat, M.A.; Khan, M.A.; Noor, T. A Non-enzymatic Electrochemical Sensor for Glucose Detection Based on Ag@TiO2@ Metal-Organic Framework (ZIF-67) Nanocomposite. Front. Chem. 2020, 8, 573510. [Google Scholar] [CrossRef]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Qin Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, M.; Xiong, C.; Zhu, X.; Chen, C.; Zhou, F.; Dong, Y.; Wang, Y.; Xu, J.; Li, Y.; et al. Dual confinement of high–loading enzymes within metal–organic frameworks for glucose sensor with enhanced cascade biocatalysis. Biosens. Bioelectron. 2022, 196, 113695. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Li, J.; Miao, F.; Zang, Y. Carbon Cloth Loaded NiCo2O4 Nano-Arrays to Construct Co-MOF@GO Nanocubes: A High-Performance Electrochemical Sensor for Non-Enzymatic Glucose. IEEE Sens. J. 2022, 22, 13898–13907. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Wang, J. Facile synthesis of bimetallic metal–organic frameworks on nickel foam for a high performance non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 904, 115887. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Liu, Y.; Yuan, G.; Zhang, S.; Xu, L.; Xue, H.; Pang, H. Turning coordination environment of 2D nickel-based metal-organic frameworks by π-conjugated molecule for enhancing glucose electrochemical sensor performance. Mater. Today Chem. 2022, 24, 100885. [Google Scholar] [CrossRef]
- Vignesh, A.; Vajeeston, P.; Pannipara, M.; Al-Sehemi, A.G.; Xia, Y.; Gnana Kumar, G. Bimetallic metal-organic framework derived 3D hierarchical NiO/Co3O4/C hollow microspheres on biodegradable garbage bag for sensitive, selective, and flexible enzyme-free electrochemical glucose detection. Chem. Eng. J. 2022, 430, 133157. [Google Scholar] [CrossRef]
- Wei, X.; Liu, N.; Chen, W.; Qiao, S.; Chen, Y. Three-phase composites of NiFe2O4/Ni@C nanoparticles derived from metal-organic frameworks as electrocatalysts for the oxygen evolution reaction. Nanotechnology 2021, 32, 175701. [Google Scholar] [CrossRef]
- Dong, S.; Niu, H.; Sun, L.; Zhang, S.; Wub, D.; Yang, Z.; Xiang, M. Highly dense Ni-MOF nanoflake arrays supported on conductive graphene/carbon fiber substrate as flexible microelectrode for electrochemical sensing of glucose. J. Electroanal. Chem. 2022, 911, 116219. [Google Scholar] [CrossRef]
- Zhai, X.; Cao, Y.; Sun, W.; Cao, S.; Wang, Y.; Hea, L.; Yao, N.; Zhao, D. Core-shell composite N-doped-Co-MOF@polydopamine decorated with Ag nanoparticles for non-enzymatic glucose sensors. J. Electroanal. Chem. 2022, 918, 116491. [Google Scholar] [CrossRef]
- Shu, Y.; Shang, Z.; Su, T.; Zhang, S.; Lu, Q.; Xu, Q.; Hu, X. A highly flexible Ni–Co MOF nanosheet coated Au/PDMS film based wearable electrochemical sensor for continuous human sweat glucose monitoring. Analyst 2022, 147, 1440–1448. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Long, Z.; Zou, J.; Luo, L.; Zhou, X.; Liu, H.; He, W.; Shen, K.; Wu, J. A Glucose Sensor Based on Surface Functionalized MXene. IEEE Trans. Nanotechnol. 2022, 21, 399–405. [Google Scholar] [CrossRef]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A.K. Electrochemical Sensing of Glucose Using Glucose Oxidase/PEDOT:4-Sulfocalix [4]arene/MXene Composite Modified Electrode. Micromachines 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Asaduzzaman, M.; Kim, D.K.; Jeong, S.; Pradhan, G.B.; Shin, Y.D.; Yoon, S.H.; Sharma, S.; et al. A Nanoporous Carbon-MXene Heterostructured Nanocomposite-Based Epidermal Patch for Real-Time Biopotentials and Sweat Glucose Monitoring. Adv. Funct. Mater. 2022, 32, 2208344. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, M.; Dong, H.; Li, T.; Zang, X.; Li, X.; Ni, Z. Free-standing MXene/chitosan/Cu2O electrode: An enzyme-free and efficient biosensor for simultaneous determination of glucose and cholesterol. J. Zhejiang Univ.-Sci. A Appl. Phys. Eng. 2022, 23, 579–586. [Google Scholar]
- Han, X.; Cao, K.; Yao, Y.; Zhao, J.; Chai, C.; Dai, P. A novel electrochemical sensor for glucose detection based on a Ti3C2Tx/ZIF-67 nanocomposite. RSC Adv. 2022, 12, 20138. [Google Scholar] [CrossRef]
- Kumar, V.; Shukla, S.K.; Choudhary, M.; Gupta, J.; Chaudhary, P.; Srivastava, S.; Kumar, M.; Kumar, M.; Kumar Sarma, D.; Yadav, B.C.; et al. Ti2C-TiO2 MXene Nanocomposite-Based High-Efficiency Non-Enzymatic Glucose Sensing Platform for Diabetes Monitoring. Sensors 2022, 22, 5589. [Google Scholar] [CrossRef]
- Manoj, D.; Aziz, A.; Muhammad, N.; Wang, Z.; Xiao, F.; Asif, M.; Sun, Y. Integrating Co3O4 nanocubes on MXene anchored CFE for improved electrocatalytic activity: Freestanding flexible electrode for glucose sensing. J. Environ. Chem. Eng. 2022, 10, 108433. [Google Scholar] [CrossRef]
- Gopal, T.S.; Jeong, S.K.; Alrebdi, T.A.; Pandiaraj, S.; Alodhayb, A.; Muthuramamoorthy, M.; Grace, A.N. MXene-based composite electrodes for efficient electrochemical sensing of glucose by non-enzymatic method. Mater. Today Chem. 2022, 24, 100891. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Shang, S.; Gao, W.; Wang, X.; Hong, J.; Hua, C.; You, Z.; Liu, Y.; Chen, J. Recrystallization of 2D C-MOF Films for High-Performance Electrochemical Sensors. ACS Appl. Mater. Interfaces 2023, 15, 16991–16998. [Google Scholar] [CrossRef]
- Venkata Ratnam, K.; Manjunatha, H.; Janardan, S.; Chandra Babu Naidu, K.; Ramesh, S. Nonenzymatic electrochemical sensor based on metal oxide, MO (M¼ Cu, Ni, Zn, and Fe) nanomaterials for neurotransmitters: An abridged review. Sens. Int. 2020, 1, 100047. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Jayalatha Kulandaisamy, A.; Balaguru Rayappan, J.B. Principles and recent developments in optical and electrochemical sensing of dopamine: A comprehensive review. Measurement 2021, 183, 109873. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Filip, J.; Jerigova, M.; Velic, D.; Kasak, P.; Mahmoud, K.A.; Tkac, J. Highly stable Ti3C2Tx (MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications. Sens. Actuators B 2018, 263, 360–368. [Google Scholar] [CrossRef]
- Lei, Y.; Butler, D.; Lucking, M.C.; Zhang, F.; Xia, T.; Fujisawa, K.; Granzier-Nakajima, T.; Cruz-Silva, R.; Endo, M.; Terrones, H.; et al. Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci. Adv. 2020, 6, abc4250. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Sabar, M.; Amara, U.; Riaz, S.; Hayat, A.; Nasir, M.; Nawaz, M.H. Fabrication of MoS2 enwrapped carbon cloth as electrochemical probe for non-enzymatic detection of dopamine. Mater. Lett. 2022, 308, 131233. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Wu, N.; Zhang, J.; Zhang, T.; Liu, B.; Pan, Z.; Liping Peng, L.; Yang, W. A novel triazine-based covalent organic framework combined with AuNPs and reduced graphene oxide as an electrochemical sensing platform for the simultaneous detection of uric acid, dopamine and ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127928. [Google Scholar] [CrossRef]
- Liu, X.; Cui, G.; Dong, L.; Wang, X.; Zhen, Q.; Sun, Y.; Ma, S.; Zhang, C.; Pang, H. Synchronous electrochemical detection of dopamine and uric acid by a PMo12@MIL-100(Fe)@PVP nanocomposite. Anal. Biochem. 2022, 648, 114670. [Google Scholar] [CrossRef]
- Masood, T.; Asad, M.; Riaz, S.; Akhtar, N.; Hayat, A.; Shenashen, M.A.; Rahman, M.M. Non-enzymatic electrochemical sensing of dopamine from COVID-19 quarantine person. Mater. Chem. Phys. 2022, 289, 126451. [Google Scholar] [CrossRef]
- Amara, U.; Sarfraz, B.; Mahmood, K.; Taqi Mehran, M.; Muhammad, N.; Hayat, A.; Hasnain Nawaz, M. Fabrication of ionic liquid stabilized MXene interface for electrochemical dopamine detection. Microchim. Acta 2022, 189, 64. [Google Scholar] [CrossRef]
- Wen, M.; Xing, Y.; Liu, G.; Hou, S.; Hou, S. Electrochemical sensor based on Ti3C2 membrane doped with UIO-66-NH2 for dopamine. Microchim. Acta 2022, 189, 141. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, S.; Liu, S.; Tong, Z.; Wang, X.; Xu, X. Preparation of ZnO/Ti3C2Tx/Nafion/Au electrode. Microchem. J. 2022, 175, 107068. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A high-sensitive dopamine electrochemical sensor based on multilayer Ti3C2 MXene, graphitized multi-walled carbon nanotubes and ZnO nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Arbabi, N.; Beitollahi, H. Ti3C2 Nano Layer Modified Screen Printed Electrode as a Highly Sensitive Electrochemical Sensor for the Simultaneous Determination of Dopamine and Tyrosine. Surf. Eng. Appl. Electrochem. 2022, 58, 13–19. [Google Scholar] [CrossRef]
- Sun, X.; Chen, C.; Xiong, C.; Zhang, C.; Zheng, X.; Wang, J.; Gao, X.; Yu, Z.-Q.; Wu, Y. Surface modification of MoS2 nanosheets by single Ni atom for ultrasensitive dopamine detection. Nano Res. 2023, 16, 917–924. [Google Scholar] [CrossRef]
- Ji, S.F.; Chen, Y.J.; Wang, X.L.; Zhang, Z.D.; Wang, D.S.; Li, Y.D. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900–11955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Li, G.L.; Chen, G.; Wu, D.; Zhou, X.X.; Wu, Y.N. Single-atom nanozymes: A rising star for biosensing and biomedicine. Coord. Chem. Rev. 2020, 418, 213376. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2-based advanced biosensors: A review. ACS Appl. Nano Mater. 2018, 1, 2–25. [Google Scholar] [CrossRef]
- Pavličková, M.; Lorencová, L.; Hatala, M.; Kováč, M.; Tkáč, J.; Gemeiner, P. Facile fabrication of screen-printed MoS2 electrodes for electrochemical sensing of dopamine. Sci. Rep. 2022, 12, 11900. [Google Scholar] [CrossRef]
- Peaston, R.T.; Weinkove, C. Measurement of catecholamines and their metabolites. Ann. Clin. Biochem. 2004, 41, 17–38. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Chen, Y.; Yang, J.; Dong, J.; Lu, X. L-Cysteine-Terminated Triangular Silver Nanoplates/MXene Nanosheets are Used as Electrochemical Biosensors for Efficiently Detecting 5-Hydroxytryptamine. Anal. Chem. 2021, 93, 16655–16663. [Google Scholar] [CrossRef]
- Kim, H.-U.; Koyappayil, A.; Seok, H.; Aydin, K.; Kim, C.; Park, K.-Y.; Jeon, N.; Seok Kang, W.; Lee, M.-H.; Kim, T. Concurrent and Selective Determination of Dopamine and Serotonin with Flexible WS2/Graphene/Polyimide Electrode Using Cold Plasma. Small 2021, 17, 2102757. [Google Scholar] [CrossRef]
- Jiang, M.; Tian, L.; Su, M.; Cao, X.; Jiang, Q.; Huo, X.; Yu, C. Real-time monitoring of 5-HT release from cells based on MXene hybrid single-walled carbon nanotubes modified electrode. Anal. Bioanal. Chem. 2022, 414, 7967–7976. [Google Scholar] [CrossRef]
- Su, M.; Lan, H.; Tian, L.; Jiang, M.; Cao, X.; Zhu, C.; Yu, C. Ti3C2Tx-reduced graphene oxide nanocomposite-based electrochemical sensor for serotonin in human biofluids. Sens. Actuators B Chem. 2022, 367, 132019. [Google Scholar] [CrossRef]
- Stárka, L.; Dušková, M. What Is a Hormone? Physiol. Res. 2020, 69, S183–S185. [Google Scholar] [CrossRef]
- Burcu Bahadır, E.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Zea, M.; Bellagambi, F.C.; Halima, H.B.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical sensors for cortisol detections: Almost there. Trends Anal. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 2022, 203, 114039. [Google Scholar] [CrossRef]
- San Nah, J.; Chandra Barman, S.; Abu Zahed, M.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Yeong Park, J. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar]
- Hernandez, P.; Sanchez, I.; Paton, F.; Hernandez, L. Cyclic voltammetry determination of epinephrine with a carbon fiber ultramicroelectrode. Talanta 1998, 46, 985–991. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Yue, H.; Gao, X.; Huang, S.; Zhang, X.; Yu, Y.; Zhang, H.; Zhang, H. Electrochemical determination of epinephrine based on Ti3C2Tx MXene-reduced graphene oxide/ITO electrode. J. Electroanal. Chem. 2021, 895, 115425. [Google Scholar] [CrossRef]
- Lin, J.; Lu, H.; Duan, Y.; Yu, C.; Li, L.; Ding, Y. Fabrication of bimetallic ZIF/carbon nanofibers composite for electrochemical sensing of adrenaline. J. Mater. Sci. 2022, 57, 6629–6639. [Google Scholar] [CrossRef]
- Singh, V.; Krishnan, S. An Electrochemical Mass Sensor for Diagnosing Diabetes in Human Serum. Analyst 2014, 139, 724–728. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, L.-Y.; Duann, Y.-F.; Chen, H.-H.; Su, C.; Liu, X.; He, J.-H.; Chung, R.-J. MOF-Derived Cu-BTC Nanowire-Embedded 2D Leaf-like Structured ZIF Composite-Based Aptamer Sensors for Real-Time In Vivo Insulin Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 28639–28650. [Google Scholar] [CrossRef]
- Balloux, F.; van Dorp, L. Q&A: What are pathogens, and what have they done to and for us? BMC Biol. 2017, 15, 91. [Google Scholar]
- Wang, B.; Wang, H.; Lu, X.; Zheng, X.; Yang, Z. Recent Advances in Electrochemical Biosensors for the Detection of Foodborne Pathogens: Current Perspective and Challenges. Foods 2023, 12, 2795. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, X.; Wu, J.; Liu, Q.; Li, D.; Huang, S.; Xie, H.; Yu, Z.; Gan, N. Reusable electrochemical biosensing platform based on egg yolk antibody-labeled magnetic covalent organic framework for on-site detection of Escherichia coli in foods. Sens. Actuators B Chem. 2022, 369, 132320. [Google Scholar] [CrossRef]
- Skorjanc, T.; Mavrič, A.; Nybo Sørensen, M.; Mali, G.; Wu, C.; Valant, M. Cationic Covalent Organic Polymer Thin Film for Label-free Electrochemical Bacterial Cell Detection. ACS Sens. 2022, 7, 2743–2749. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous organic polymers as a platform for sensing applications. Chem. Soc. Rev. 2022, 51, 2031. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, Y.; Lun, K.; Wu, B.; He, L.; Wang, M.; Fang, S.; Zhang, Z.; Zhou, L. Sensitive detection of Escherichia coli in diverse foodstuffs by electrochemical aptasensor based on 2D porphyrin-based COF. Microchim. Acta 2023, 190, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Jia, X.; Liu, J.; Sun, M.; Wu, J. Rapid and simple preparation of an MXene/polypyrrole-based bacteria imprinted sensor for ultrasensitive Salmonella detection. J. Electroanal. Chem. 2022, 918, 116513. [Google Scholar] [CrossRef]
- Salimiyan Rizi, K.; Hatamluyi, B.; Darroudi, M.; Meshkat, Z.; Aryan, E.; Soleimanpour, S.; Rezayi, M. PCR-free electrochemical genosensor for Mycobacterium tuberculosis complex detection based on two-dimensional Ti3C2 Mxene-polypyrrole signal amplification. Microchem. J. 2022, 179, 107467. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Wei, W.; Lin, H.; Hao, T.; Su, X.; Jiang, X.; Wang, S.; Hu, Y.; Guo, Z. Dual-mode ECL/SERS immunoassay for ultrasensitive determination of Vibrio vulnificus based on multifunctional MXene. Sens. Actuators B Chem. 2021, 332, 129525. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, S.; Jia, Z.; Xie, H.; Li, T.; Wang, Q.; Gan, N. A dual-mode aptasensor for foodborne pathogens detection using Pt, phenylboric acid and ferrocene modified Ti3C2 MXenes nanoprobe. Sens. Actuators B Chem. 2022, 351, 130839. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Bianco, A.; Kalantar-Zadeh, K. Two-Dimensional Material-Based Biosensors for Virus Detection. ACS Sens. 2020, 5, 3739–3769. [Google Scholar] [CrossRef]
- Sheikhzadeh, E.; Beni, V.; Zourob, M. Nanomaterial application in bio/sensors for the detection of infectious diseases. Talanta 2021, 230, 122026. [Google Scholar] [CrossRef]
- Viana Ribeiro, B.; Reis Cordeiro, T.A.; Ramos Oliveira Freitas, G.; Ferreira, L.F.; Leoni Franco, D. Biosensors for the detection of respiratory viruses: A review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef] [PubMed]
- Yugender Goud, K.; Koteshwara Reddy, K.; Khorshed, A.; Sunil Kumar, V.; Mishra, R.K.; Oraby, M.; Hatem Ibrahim, A.; Kim, H.; Vengatajalabathy Gobid, K. Electrochemical diagnostics of infectious viral diseases: Trends and challenges. Biosens. Bioelectron. 2021, 180, 113112. [Google Scholar]
- Manring, N.; Ahmed, M.M.N.; Tenhoff, N.; Smeltz, J.L.; Pathirathna, P. Recent Advances in Electrochemical Tools for Virus Detection. Anal. Chem. 2022, 94, 7149–7157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef]
- Tian, J.; Liang, Z.; Hu, O.; He, O.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Gumus, Z.P.; Soylak, M.; Erk, N. Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta 2022, 240, 123211. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M. SARS-CoV-2 virus label-free electrochemical nanohybrid MIP-aptasensor based on Ni3(BTC)2 MOF as a high-performance surface substrate. Microchim. Acta 2022, 189, 287. [Google Scholar] [CrossRef]
- Panda, S.; Deshmukh, K.; Mustansar Hussain, C.; Khadheer Pasha, S.K. 2D MXenes for combatting COVID-19 Pandemic: A perspective on latest developments and innovations. FlatChem 2022, 33, 100377. [Google Scholar] [CrossRef]
- Yenyu Chen, W.; Hang Lin, H.; Kumar Barui, A.; Ulloa Gomez, A.M.; Wendt, M.K.; Stanciu, L.A. DNA-Functionalized Ti3C2Tx MXenes for Selective and Rapid Detection of SARS-CoV-2 Nucleocapsid Gene. ACS Appl. Nano Mater. 2022, 5, 1902–1910. [Google Scholar] [CrossRef]
- Lin, X.; Lian, X.; Luo, B.; Huang, X.-C. A highly sensitive and stable electrochemical HBV DNA biosensor based on ErGO-supported Cu-MOF. Inorg. Chem. Commun. 2020, 119, 108095. [Google Scholar] [CrossRef]
- Rezki, M.; Septiani, N.L.W.; Iqbal, M.; Harimurti, S.; Sambegoro, P.; Damar Rastri Adhika, D.R.; Yuliarto, B. Amine-functionalized Cu-MOF nanospheres towards label-free hepatitis B surface antigen electrochemical immunosensors. J. Mater. Chem. B 2021, 9, 5711–5721. [Google Scholar] [CrossRef]
- Biomarkers Definition Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Bolandi, N.; Hemmati, H.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Oroojalian, F.; Reza Majidi, M.; de la Guardia, M.; Mokhtarzadeh, A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review. Microchem. J. 2022, 177, 107248. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, J.; Liang, Y.; Tian, F.; Peng, L.; Huo, D.; Hou, C. Functionalized Carbon Nanotube-Decorated MXene Nanosheet-Enabled Microfluidic Electrochemical Aptasensor for Carcinoembryonic Antigen Determination. ACS Sustain. Chem. Eng. 2021, 9, 15386–15393. [Google Scholar] [CrossRef]
- Wang, Q.; Xin, H.; Wang, Z. Label-Free Immunosensor Based on Polyaniline-Loaded MXene and Gold-Decorated β-Cyclodextrin for Efficient Detection of Carcinoembryonic Antigen. Biosensors 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, H.; Yuan, R.; Xiang, Y. Trimetallic nanoparticle-decorated MXene nanosheets for catalytic electrochemical detection of carcinoembryonic antigen via Exo III-aided dual recycling amplifications. Sens. Actuators B Chem. 2022, 359, 131617. [Google Scholar] [CrossRef]
- Wang, L.; Xie, H.; Lin, Y.; Wang, M.; Sha, L.; Yu, X.; Yang, J.; Zhao, J.; Li, G. Covalent organic frameworks (COFs)-based biosensors for the assay of disease biomarkers with clinical applications. Biosens. Bioelectron. 2022, 217, 114668. [Google Scholar] [CrossRef]
- Qiu, R.; Mu, W.; Wu, C.; Wu, M.; Feng, J.; Rong, S.; Ma, H.; Chang, D.; Pan, H. Sandwich-type immunosensor based on COF-LZU1 as the substrate platform and graphene framework supported nanosilver as probe for CA125 detection. J. Immunol. Methods 2022, 504, 113261. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Ning, G.; Sun, W.; Wang, L.; Liang, H.; Xu, H.; He, C.; Zhao, H.; Li, C.-P. A novel affinity peptide–antibody sandwich electrochemical biosensor for PSA based on the signal amplification of MnO2-functionalized covalent organic framework. Talanta 2021, 233, 122520. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Liu, J.; Zhou, N.; Zhang, C.; He, L.; Zhang, Z.; Liu, Z. Two-dimensional porphyrin-based covalent organic framework: A novel platform for sensitive epidermal growth factor receptor and living cancer cell detection. Biosens. Bioelectron. 2019, 126, 734–742. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, L.; Wei, X.; Sun, J.; Xu, D. Detection of antibiotics by electrochemical sensors based on metal-organic frameworks and their derived materials. Microchem. J. 2022, 183, 107946. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Liu, J.; Guo, C.; Peng, D.; Jia, Q.; He, L.; Zhang, Z.; Du, M. Covalent organic framework-based electrochemical aptasensors for the ultrasensitive detection of antibiotics. Biosens. Bioelectron. 2019, 132, 8–16. [Google Scholar] [CrossRef]
- Ma, X.; Pang, C.; Li, S.; Xiong, Y.; Li, J.; Luo, J.; Yang, Y. Synthesis of Zr-coordinated amide porphyrin-based two-dimensional covalent organic framework at liquid-liquid interface for electrochemical sensing of tetracycline. Biosens. Bioelectron. 2019, 146, 111734. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, J.; Yao, L.; Xue, F.; Lu, J.; Li, B.; Chen, W. Aptamer-mediated colorimetric method for rapid and sensitive detection of chloramphenicol in food. Food Chem. 2018, 260, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-K.; Ye, H.-L.; Zhao, X.-X.; Sun, X.-L.; Zhu, Q.-Q.; Han, Z.-Y.; Yuan, R.; He, H. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, L.; Ren, J.; Cui, M.; Li, N.; Zhao, H.; Qu, Y.; Ji, X. Facile synthesis of surface functionalized Pd2+@P-CDP/COFs for highly sensitive detection of norfloxacin drug based on the host-guest interaction. J. Pharm. Biomed. Anal. 2022, 219, 114956. [Google Scholar] [CrossRef] [PubMed]
- Cháfer-Pericás, C.; Maquieira, A.; Puchades, R. Fast screening methods to detect antibiotic residues in food samples. TrAC Trends Anal. Chem. 2010, 29, 1038–1049. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, H.; Xu, L.; Waterhouse, G.I.N.; Zhang, H.; Qiao, X.; Xu, Z. Ultrasensitive determination of sulfathiazole using a molecularly imprinted electrochemical sensor with CuS microflowers as an electron transfer probe and Au@COF for signal amplification. Food Chem. 2020, 332, 127376. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Settu, R.; Chen, S.M.; Chen, T.W. Voltammetric sensing of sulfamethoxazole using a glassy carbon electrode modified with a graphitic carbon nitride and zinc oxide nanocomposite. Microchim Acta 2018, 185, 396. [Google Scholar] [CrossRef]
- Shahsavari, M.; Tajik, S.; Sheikhshoaie, I.; Beitollahi, H. Fabrication of Nanostructure Electrochemical Sensor Based on the Carbon Paste Electrode (CPE) Modified With Ionic Liquid and Fe3O4/ZIF-67 for Electrocatalytic Sulfamethoxazole Detection. Top. Catal. 2022, 65, 577–586. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Guo, F.; Tian, G.; Fan, C.; Zong, Z.; Wang, J.; Xu, J. A zirconium–organic framework nanosheet-based aptasensor with outstanding electrochemical sensing performance. Inorg. Chem. Commun. 2022, 145, 109970. [Google Scholar] [CrossRef]
- Li, H.-K.; An, Y.-X.; Zhang, E.-H.; Zhou, S.-N.; Li, M.-X.; Li, Z.-J.; Li, X.; Yuan, R.; Zhang, W.; He, H. A covalent organic framework nanosheet-based electrochemical aptasensor with sensitive detection performance. Anal. Chim. Acta 2022, 1223, 340204. [Google Scholar] [CrossRef]



















| Electrode | 2D Nanomaterial | Format | Technique | Sample | Linearity (μM) | LOD (μM) | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| GCE | ZIF-67 | sensor based on Ag@ZIF-67 nanocomposite | A | - | 2–1000 | 0.66 | - | - | [50] |
| GCE | ZIF-67 | sensor based on Ag@TiO2@ZIF-67 nanocomposite | A | - | 48–1000 | 0.99 | - | - | [51] |
| rGO/PU fiber | Ni–Co MOF | sensor based on Ni–Co MOF/Ag nanocomposite | A | Human sweat | 10–660 | 3.28 | - | Glucometer blood test | [52] |
| SPCPE | NC-ZIF | biosensor based on GOX/Hemin@NC-ZIF | A | Human sweat | 50–600 | 2 | - | Glucometer blood test | [53] |
| CC | ZIF-67 | sensor based on ZIF-67@GO/NiCo2O4 | A | - | 0.3–157.4 | 0.16 | - | - | [54] |
| NF | Cu1Co2-MOF | sensor based on Cu1Co2-MOF | CA | - | 50–500 | 23 | - | - | [55] |
| GCE | Ni-MOF | sensor based on Ni-MOF@Ni-HHTP-5 NSs core@shell structures | A | - | 500–2,665,500 | 48.5 | - | - | [56] |
| BCSB | Ni-Co MOF as sacrificial template | sensor based on NiO/Co3O4/C nanocomposite | A | Human blood serum | 0.2–10,000 | 0.045 | 98.3–102.4 | - | [57] |
| CFE | Ni-MOF | sensor based on Ni-MOF/rGO nanocomposite | A | Orange juice | 6–2090 | 0.6 | - | - | [59] |
| GCE | N-doped-Co- MOF | sensor based on N-Co-MOF@PDA-Ag nanocomposite | A | Human serum | 1–2000 | 0.5 | 96–110 | - | [60] |
| AuE | Ni-Co-MOF | sensor based on Ni–Co MOF/Au/PDMS nanocomposite | A | Human sweat | 20–790 | 4.25 | - | Glucometer blood test | [61] |
| SPCE | MXene (Ti3C2Tx) | biosensor based on ZnO TPs/MXene/GOX | CA | Human sweat | 50–700 | 17 | - | Glucometer blood test | [62] |
| Cr/AuE | MXene (Ti3C2Tx) | biosensor based on MXene/GOX | A | - | 100–10,000 | 12.1 | - | - | [63] |
| GCE | MXene (Ti3C2Tx) | biosensor based on PEDOT:SCX/MXene/GOX | A | Fruit juice | 500–8000 | 22.5 | 96–99 | - | [64] |
| AuE | MXene (Ti3C2Tx) | biosensor based on GOX/PtNPs/NPC/MXene | CA | Human sweat | 3–21,000 | 7 | - | Glucometer blood test | [65] |
| GCE | MXene | sensor based on MXene/CHI/Cu2O | CV | Human serum | 52.4–2000 | 52.4 | 98.04–102.94 | - | [66] |
| GCE | MXene (Ti3C2Tx) ZIF-67 | sensor based on Ti3C2Tx/ZIF-67 nanocomposite | A | - | 5–7500 | 3.81 | - | - | [67] |
| GCE | Ti2C-MXene | sensor based on Ti2C-TiO2-MXene nanocomposite | DPV | Human serum | 0.1–200 | 0.12 | 99.80–100.23 | Glucometer blood test | [68] |
| CFE | MXene | sensor based on Co3O4/MXene nanocomposite | A | Human blood serum, urine | 0.05–7440 | 0.010 | 97.80–101.60 | Glucometer blood test | [69] |
| GCE | MXene (Ti3C2Tx) | sensor based on MXene-Cu2O nanocomposite | CA | Human serum | 10–30,000 | 2.83 | - | Glucometer blood test | [70] |
| AuE | c-MOF (Cu3(HHTP)2) | sensor based on c-MOF | A | - | 0.2–7 mM | 10 | - | - | [71] |
| Electrode | 2D Nanomaterial | Format | Technique | Sample | Linearity | LOD | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | MXene (Ti3C2Tx) | sensor based on Ti3C2Tx/PtNPs | A | - | Up to 750 μM | 10 nM | - | [74] |
| PGS | TMD (MoS2) | sensor based on MoS2 | DPV | DA | 0.05–5 nM 5 nM–5 mM | 50 pM | - | [75] |
| GCE | MXene (Ti-C-Tx) | sensor based on Ti-C-Tx | DPV | DA,AA,UA | DA: 0.5–4 μM AA: 0.5–50 μM UA: 0.1–1.5 μM | DA: 0.06 μM AA: 4.6 μM UA: 0.075 μM | - | [76] |
| CC | TMD (MoS2) | sensor based on MoS2 nanosheets | CV | DA | 250–4000 μM | 0.3 μM | - | [77] |
| GCE | TS-COF | sensor based on AuNPs@TS-COF/rGO | DPV | DA,AA,UA in human urine | DA: 20–100 μM AA: 8–900 μM UA: 25–80 μM | DA: 0.03 μM AA: 4.30 μM UA: 0.07 μM | 97–104.4 | [78] |
| GCE | Fe-based MOF MIL-100(Fe) | sensor based on POM- MOF/PVP | DPV | DA,UA in human serum | DA: 1–247 μM UA: 5–406 μM | DA: 1 μM UA: 5 μM | DA: 97.67–102.16 UA: 97.81–102.89 | [79] |
| LPE | MOF (ZIF-67) | sensor based on ZIF-67/PEDOT | A | DA in human blood | 15–240 μM | 0.04 μM | - | [80] |
| GPE | MXene (Ti3C2Cl2) | sensor based on IL-MXene | A | DA in human serum | 100–2000 μM | 702 nM | 98.3–100 | [81] |
| GCE | MXene (Ti3C2) MOF (UIO-66-NH2) | sensor based on Ti3C2/UIO-66-NH2 | DPV | DA in human serum | 1–250 fM | 0.81 fM | 101.2–103.5 | [82] |
| AuE | MXene (Ti3C2Tx) | sensor based on ZnO NPs/Ti3C2Tx | CA | DA in human serum | 0.1–1200 μM | 0.076 μM | 97.8–102.2 | [83] |
| GCE | MXene (Ti3C2) | sensor based on Ti3C2/g-MWCNTs/ ZnO NSPs | DPV | DA in human serum | 0.01–30 μM | 3.2 nM | 98.6–105.9 | [84] |
| SPCE | MXene (Ti3C2) | sensor based on Ti3C2 | DPV | DA in human urine Tyr in drug | 0.5–600 μM | 0.15 μM | DA: 97.1–104.0 Tyr: 96.7–102.5 | [85] |
| GCE | TMD (MoS2) | sensor based on Ni-MoS2 | DPV | DA in bovine serum | 1 pM–1 mM | 1 pM | 97.0–105.0 | [87] |
| FTO SPE | TMD (MoS2) | sensor based on MoS2 | DPV | DA | Up to 300 μM | 260 nM | - | [90] |
| GCE | MXene (Ti3C2Tx) | sensor based on Tri-AgNPs/L-Cys/ MXene | DPV | 5-HT in human serum | 0.5–150 μM | 0.08 μM | 95.38–102.3 | [92] |
| WGPE | TMD (WS2) | sensor based on WGP | DPV | DA, 5-HT in artificial CSF | DA: 1.21–13.37 μM 5-HT: 9.9–0.249 μM | DA: 1.24 μM 5-HT: 0.24 μM | 95.2–104.1 | [93] |
| GCE | MXene (Ti3C2) | sensor based on MXene/SWCNTs | CA | 5-HT produced by living cells | 0.004–103.2 μM | 1.5 nM | - | [94] |
| GCE | MXene (Ti3C2) | sensor based on MXene/rGO | DPV | 5-HT in human plasma | 0.025–147 μM | 10 nM | 96.4–107 | [95] |
| Electrode | 2D Nanomaterial | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Thread Conductive E | MXene | immunosensor immobilizing anti-cortisol on L-cys/AuNPs/ MXene | A | Cortisol in sweat | 5–180 ng mL−1 | 0.54 ng mL−1 | 94.47–102 | - | [99] |
| LBG/PDMS | MXene (Ti3C2Tx) | immunosensorimmobilizing anti-cortisol on Ti3C2Tx/LBG/ PDMS | EIS | Cortisol in sweat | 0.01–100 nM | 3.88 pM | 93.68–99.1 | - | [100] |
| ITOE | MXene (Ti3C2Tx) | sensor based on GMA | DPV | EP in human urine | 1–60 μM | 3.5 nM | 95.7–105.7 | - | [102] |
| GCE | MOF (CoMn-ZIF) | sensor based on CoMnZIF-CNF | DPV | EP in human urine | 5–1000 μM | 1.667μM | 97.51–102.53 | - | [103] |
| DGE | MOF (ZIF-L) | aptasensor immobilizing insulin aptamer on Cu-BTC/ZIF-L | DPV | Insulin in human serum | 0.1 pM–5 μM | 0.027 pM | 97.2–98.5 | ELISA | [105] |
| Electrode | 2D Nanomaterial | Format | Technique | Sample | Linearity | LOD | Recovery (%) | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| SPCE | m-COF | immunosensor immobilizing IgY antibody on m-COF | SWV | E. coli in milk, beef, shrimps | 10–108 CFU mL−1 | 3 CFU mL−1 | 90–103 | ELISA | [108] |
| IDEA | COP (CATN) | sensor based on CATN | EIS | E. coli | Up to 10 CFU mL−1 | 2 CFU mL−1 | - | - | [109] |
| AuE | COF (Tph-TDC-COF) | aptasensor based on E. coli aptamer immobilized on Tph-TDC-COF | EIS/DPV | E. coli in bread, milk | 10–108 CFU mL−1 | 0.17 CFU mL−1 (EIS) 0.38 CFU mL−1 (DPV) | 100.09–103.97 (bread) 99.61–100.71 (milk). | - | [111] |
| GCE | MXene | sensor based on MPBIP | EIS | Salmonella in drinking water | 103–107 CFU mL−1 | 23 CFU mL−1 | 96–109.4 | - | [112] |
| GCE | MXene (Ti3C2) | genosensor immobilizing ssDNA on PPY/MXene | DPV | M.tb in human sputum | 100 fM- 25 nM | 11.24 fM | 90.52–100.8 | PCR | [113] |
| MGCE | MXene (Ti3C2Tx) | ECL immunosensor based on Fe3O4@Ab1-VV-R6G-Ti3C2Tx@AuNRs-Ab2/ABEI | ECL | VV in seawater | 1–108 CFU mL−1 | 1 CFU mL−1 | 94.8–110.3 | - | [115] |
| AuSPE | MXene | sandwich-type aptasensor involving PBA-Fc@Pt@MXenes | DPV | VP in shrimps | 10–108 CFU mL−1 | 5 CFU mL−1 | 95.0–104.3 | - | [116] |
| AuE | MOF (MIL-53 (Al)) | sandwich-type aptasensor using 2 aptamers immobilized on AuE and Au@Pt/MIL-53 (Al) nanocomposite modified with HRP and hemin/Gquadruplex DNAzyme as signal nanoprobe | DPV | SARS-CoV-2 NP | 0.025–50 ng mL−1 | 8.33 pg mL−1 | 92–110 | ELISA | [123] |
| SPCE | MOF (SiO2@UIO-66) | label-free immunosensor including SiO2@UiO-66 nanocomposite | EIS | SARS-CoV-2 SP in nasal fluid samples | 100.0 fg∙mL−1–10.0 ng∙mL−1 | 100.0 fg mL−1 | 91.6–93.2 | PCR | [124] |
| SPCE | MOF Ni3(BTC)2 | label-free aptasensor using Ni3(BTC)2, SARS-CoV-2 aptamer, ePDA | EIS | SARS-CoV-2 virus in saliva, oropharyngeal swab | 10–108 PFU mL−1 | 3.3 PFU mL−1 | 98–104 | PCR | [125] |
| IGE | MXene (Ti3C2Tx) | genosensor using ssDNA/Ti3C2Tx | EIS | SARS-CoV-2 N gene | 105–109 copies mL−1 | 103 copies mL−1 | - | - | [127] |
| GCE | Cu-MOF | genosensor including Cu-MOF/ErGO | DPV | HBV in human serum, urine | 50.0 fM– 10.0 nM | 5.2 fM | 95.2–99.8 | - | [128] |
| GCE | Cu-MOF | label-free immunosensor using Cu-MOF nanospheres | DPV | HBV in human serum | 1–500 ng mL−1 | 730 pg mL−1 | 76.93–92.18 | - | [129] |
| Electrode | 2D Nanomaterial | (Bio)Sensor Format | Technique | Sample | Linearity | LOD | Recovery% | Ref. |
|---|---|---|---|---|---|---|---|---|
| SPCE | MXene (Ti3C2) | Aptasensor including He@CCNT/Ti3C2 | DPV | CEA in human serum | 10–106 pg mL−1 | 2.88 pg mL−1 | 95.29–105.19 | [133] |
| FTOE | MXene (Ti3C2) | Immunosensor including Au-β-CD/MXene@ PANI | DPV | CEA in human serum | 0.5–350 ng mL−1 | 42.9 pg mL−1 | 97.52–103.98 | [134] |
| GCE | Mxene (Ti3C2Tx) | Aptasensor involving Au-Pd-Pt/Ti3C2Tx | DPV | CEA in human serum | 1 fg mL−1 –1 ng mL−1 | 0.32 fg mL−1 | 98.8–104.1 | [135] |
| GCE | COF-LZU1 | Sandwich-type Immunosensor involving COF-LZU1, MrGOF, AgNPs | DPV | CA 125 in human serum | 0.001–40 U mL−1 | 0.00023 U mL−1 | 91.54–105.21 | [137] |
| GCE | COF BCN | Sandwich-type immunosensor involving MnO2@COF, AuPtNPs, BCN, PDA | DPV | PSA in human serum | 0.00005–10 ng mL−1 | 16.7 fg mL−1 | 98.9–100.2 | [138] |
| AuE | COF (p-COF) | Aptasensor based on EGFR aptamer immobilized on p-COF | DPV EIS | EGFR in human serum | 0.05–100 pg mL−1 | 7.54 × 10−3 pg mL−1 (EIS) 5.64 × 10−3 pg mL−1 (DPV). | 96.2–103.2 | [139] |
| AuE | COF (p-COF) | Aptasensor based on EGFR aptamer immobilized on p-COF | EIS | MCF-7/- | 5 × 102–1 × 105 cell·mL−1 | 61 cells·mL−1 | - | [139] |
| Electrode | 2D Nanomaterial | Format | Technique | Sample | Linearity | LOD | Recovery % | Reference Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AuE | Py-M-COF | Aptasensor including Py-M-COF | EIS | ENR in human serum | 0.01–2000 pg mL−1 | 6.07 fg mL−1 | 101.0–112.4 | - | [141] |
| AuE | Py-M-COF | Aptasensor including Py-M-COF | EIS | AMP in human serum | 0.001–1000 pg mL−1 | 0.04 fg mL−1 | 99.5–103.0 | - | [141] |
| GCE | Zr-amide-Por- based 2D COF | TC-MIECS | ECL | TC in milk | 5–60 pM | 2.3 pM | 94.0–103.5 | - | [142] |
| AuE | MOF (PCN-222) | Aptasensor including PCN-222/GO | EIS | CAP in milk, human serum, urine | 0.01–50 ng mL−1 | 7.04 pg mL−1 | 94.6–107.2 | - | [144] |
| GCE | COF | Electrochemical sensor based on Pd2+@P-CDPs/COFs | DPV | NF in eye drops | 0.08–7.0 µM 7.0–100.0 µM | 0.031 µM | 97.8–101.7 | HPLC | [145] |
| GCE | COF | Electrochemical sensor based on MIP/CuS/Au@COF | DPV | STZ in mutton, fodder, chicken liver, pig liver | 0.001–100,000 nM | 0.0043 nM | 83.0–107.3 | HPLC | [147] |
| CPE | MOF (ZIF-67) | Electrochemical sensor based on Fe3O4/ZIF-67/IL | DPV | Sulfamethoxazole in river, tap water, urine | 0.01–520.0 μM | 5.0 nM | 97.1–103.3 | - | [149] |
| GCE | COF | Electrochemical sensor based on GO@COF nanocomposite and MIP | DPV | SDZ/beef, pork, chicken, fodder | 0.5–200 μM | 0.16 μM | 82.0–108.0 | HPLC | [150] |
| AuE | MOF (PCN-222(Fe) NS) | Aptasensor including PCN-222 (Fe) NS | EIS | TOB/milk | 1.1 × 10−4–10.7 nM | 1.3 × 10−4 nM | 97.5–104.8 | - | [151] |
| AuE | COF (Tp-Bpy COF NS) | Aptasensor including Tp-Bpy COF NS | EIS | TOB/milk, river water | 2.1 × 10−4–10.7 nM | 6.57 fM | 95.4–105.2% milk 96.1–104.4% river | - | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, P.; Petrucci, R.; Curulli, A. Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications. Molecules 2024, 29, 172. https://doi.org/10.3390/molecules29010172
Di Matteo P, Petrucci R, Curulli A. Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications. Molecules. 2024; 29(1):172. https://doi.org/10.3390/molecules29010172
Chicago/Turabian StyleDi Matteo, Paola, Rita Petrucci, and Antonella Curulli. 2024. "Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications" Molecules 29, no. 1: 172. https://doi.org/10.3390/molecules29010172
APA StyleDi Matteo, P., Petrucci, R., & Curulli, A. (2024). Not Only Graphene Two-Dimensional Nanomaterials: Recent Trends in Electrochemical (Bio)sensing Area for Biomedical and Healthcare Applications. Molecules, 29(1), 172. https://doi.org/10.3390/molecules29010172