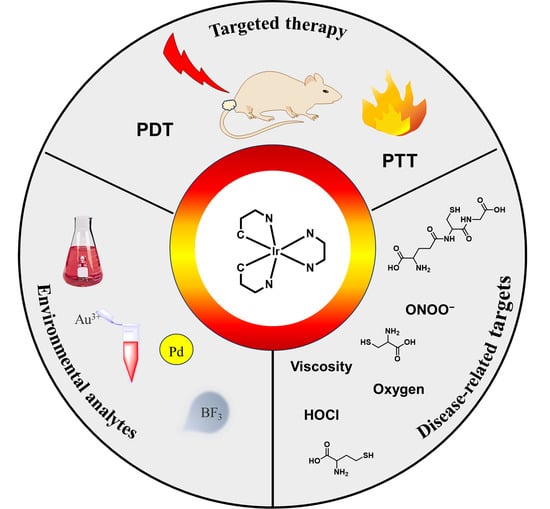
Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy
Abstract
:1. Introduction
2. General Strategies to Design NIR Iridium(III) Complexes
2.1. Cyclometalated Ligand-Enabled NIR Iridium(III) Complexes
2.1.1. Cyclometalated Ligand-Enabled NIR Iridium(III) Complexes for the Detection of Environmental Analytes
2.1.2. Cyclometalated Ligand-Enabled NIR Iridium(III) Complexes for the Detection of Disease-Related Targets
2.1.3. Cyclometalated Ligand-Enabled NIR Iridium(III) Complexes for Targeted Therapy
2.2. NIR Dye Conjugation-Enabled NIR Iridium(III) Complexes
NIR Dye Conjugation-Enabled NIR Iridium(III) Complexes for Phototherapy
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, X.W.; Pan, Y.C.; Gao, Y.F.; Song, Y.J. Recent near-infrared light-activated nanomedicine toward precision cancer therapy. J. Mater. Chem. B 2021, 9, 7076–7099. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Q.; Li, H.C.; Chen, S.; Wang, N.; Liu, G.Y.; Liu, D.J.; Ou, W.H.; Xu, F.J.; Wang, X.; Lei, D.Y.; et al. Near-infrared-activated anticancer platinum(IV) complexes directly photooxidize biomolecules in an oxygen-independent manner. Nat. Chem. 2023, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; Lavis, L.D. Caveat fluorophore: An insiders’ guide to small-molecule fluorescent labels. Nat. Methods 2022, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Kim, T.; Kim, C.; Kim, M.; Park, T. Recent Advances in Structural Design of Efficient Near-Infrared Light-Emitting Organic Small Molecules. Adv. Funct. Mater. 2023, 33, 2208082. [Google Scholar] [CrossRef]
- Martinic, I.; Eliseeva, S.V.; Petoud, S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J. Lumin. 2017, 189, 19–43. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhang, E.L.; Su, Y.P.; Cheng, T.M.; Shi, C.M. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Funovics, M.; Weissleder, R.; Tung, C.H. Protease sensors for bioimaging. Anal. Bioanal. Chem. 2003, 377, 956–963. [Google Scholar] [CrossRef]
- Sun, C.X.; Yang, J.H.; Li, L.; Wu, X.; Liu, Y.; Liu, S.F. Advances in the study of luminescence probes for proteins. J. Chromatogr. B 2004, 803, 173–190. [Google Scholar] [CrossRef]
- Bardon, K.M.; Selfridge, S.; Adams, D.S.; Minns, R.A.; Pawle, R.; Adams, T.C.; Takiff, L. Synthesis of water-soluble far-red-emitting amphiphilic BODIPY dyes. ACS Omega 2018, 3, 13195–13199. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.B.; Yang, X.H.; Peng, Z.H.; Liu, W.; Xu, J.G.; Tang, J.L.; He, X.X.; Wang, K.M. Quantum dot/methylene blue FRET mediated NIR fluorescent nanomicelles with large Stokes shift for bioimaging. Chem. Commun. 2015, 51, 14357–14360. [Google Scholar] [CrossRef]
- Harmatys, K.M.; Cole, E.L.; Smith, B.D. In vivo imaging of bone using a deep-red fluorescent molecular probe bearing multiple Iminodiacetate groups. Mol. Pharm. 2013, 10, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Patila, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Valisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.X.; Liu, X.; Pan, Y.N.; Zhang, Z.Y.; Du, M.X.; Lu, S.Y.; et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: From strategic design toward molecular imaging and theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Teets, T.S. Red to near-infrared phosphorescent Ir(III) complexes with electron-rich chelating ligands. Chem. Commun. 2021, 57, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H. Luminescent complexes of platinum, iridium, and coinage metals containing N-heterocyclic carbene ligands: Design, structural diversity, and photophysical properties. Chem. Rev. 2023, 123, 230–270. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Wang, Y.H.; Rehman, F.U.; Jiang, H.; Wang, X.M. Phosphorescent Ir(III) complexes as cellular staining agents for biomedical molecular imaging. Coord. Chem. Rev. 2020, 416, 213344. [Google Scholar] [CrossRef]
- Ning, Y.Y.; Jin, G.Q.; Wang, M.X.; Gao, S.; Zhang, J.L. Recent progress in metal-based molecular probes for optical bioimaging and biosensing. Curr. Opin. Chem. Biol. 2022, 66, 102097. [Google Scholar] [CrossRef]
- Jin, G.Q.; Guo, L.J.; Zhang, J.; Gao, S.; Zhang, J.L. Luminescent metal complexes for bioassays in the near-infrared(NIR) region. Top. Curr. Chem. 2022, 380, 31. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ding, D. Recent advances of transition Ir(III) complexes as photosensitizers for improved photodynamic therapy. VIEW 2021, 24, 20200179. [Google Scholar] [CrossRef]
- Wu, Q.; Dai, P.; Wang, Y.; Zhang, J.; Li, M.; Zhang, K.Y.; Liu, S.; Huang, W.; Zhao, Q. Time-resolved analysis of photoluminescence at a single wavelength for ratiometric and multiplex biosensing and bioimaging. Chem. Sci. 2021, 12, 11020–11027. [Google Scholar] [CrossRef]
- Wang, W.; Wu, K.-J.; Vellaisamy, K.; Leung, C.-H.; Ma, D.-L. Peptide-conjugated long-lived theranostic imaging for targeting GRPr in cancer and immune cells. Angew. Chem. Int. Ed. 2020, 59, 17897–17902. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Q.; Wu, K.-J.; Zhang, Z.; Liu, T.-M.; Ko, C.-N.; Zhu, W.-G.; Ma, D.-L.; Wang, W.; Leung, C.-H. Development of a sensitive luminescent probe to uncover new BRD4 inhibitors in living cells. Chem. Eng. J. 2023, 463, 142356. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Qiao, J. Near-infrared emitting iridium complexes: Molecular design, photophysical properties, and related applications. iScience 2021, 24, 102858. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.H.; Li, F.Y. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Farrer, N.J.; Salassa, L.; Sadler, P.J. Photoactivated chemotherapy(PACT): The potential of excited-state d-block metals in medicine. Dalton Trans. 2009, 10690–10701. [Google Scholar] [CrossRef]
- Bonnet, S. Shifting the light activation of metallodrugs to the red and near-infrared region in anticancer phototherapy. Comments Inorg. Chem. 2015, 35, 179–213. [Google Scholar] [CrossRef]
- Ouyang, J.; Tang, Z.M.; Farokhzad, N.; Kong, N.; Kim, N.Y.; Feng, C.; Blake, S.; Xiao, Y.F.; Liu, C.; Xie, T.; et al. Ultrasound mediated therapy: Recent progress and challenges in nanoscience. Nano Today 2020, 35, 100949. [Google Scholar] [CrossRef]
- Wang, L.L.; Karges, J.; Wei, F.M.; Xie, L.A.; Chen, Z.L.; Gasser, G.; Ji, L.N.; Chao, H. A mitochondria-localized iridium(III) photosensitizer for two-photon photodynamic immunotherapy against melanoma. Chem. Sci. 2023, 14, 1461–1471. [Google Scholar] [CrossRef]
- Barbieri, A.; Bandini, E.; Monti, F.; Praveen, V.K.; Armaroli, N. The Rise of Near-Infrared Emitters: Organic Dyes, Porphyrinoids, and Transition Metal Complexes. Top. Curr. Chem. 2016, 374, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Jiao, J.; Xu, W.; Zhang, M.Y.; Cui, P.; Guo, Z.Q.; Deng, Y.B.; Chen, H.B.; Sun, W.F. Highly efficient far-red/NIR-absorbing neutral Ir(III) complex micelles for potent photodynamic/photothermal therapy. Adv. Mater. 2021, 33, 2100795. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.M.; Karges, J.; Shen, J.C.; Xie, L.A.; Xiong, K.; Zhang, X.T.; Ji, L.N.; Chao, H. A mitochondria-localized oxygen self-sufficient two-photon nano-photosensitizer for ferroptosis-boosted photodynamic therapy under hypoxia. Nano Today 2022, 44, 101509. [Google Scholar] [CrossRef]
- Ke, L.B.; Wei, F.M.; Xie, L.N.; Karges, J.; Chen, Y.; Ji, L.N.; Chao, H. A biodegradable iridium(III) coordination polymer for enhanced two-photon photodynamic therapy using an apoptosis-ferroptosis hybrid pathway. Angew. Chem. Int. Ed. 2022, 61, e202205429. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiong, C.Y.; Li, B.; Du, W.L.; Li, D.D.; Ma, W.; Tian, X.H.; Tian, Y.P.; Zhang, Q. Three-photon absorption iridium(III) photosensitizers featuring aggregation induced emission. Inorg. Chem. Front. 2022, 9, 1890–1896. [Google Scholar] [CrossRef]
- Jin, C.Z.; Liang, F.Y.; Wang, J.Q.; Wang, L.L.; Liu, J.P.; Liao, X.X.; Rees, T.W.; Yuan, B.; Wang, H.; Shen, Y.; et al. Rational design of cyclometalated iridium(III) complexes for three-photon phosphorescence bioimaging. Angew. Chem. Int. Ed. 2020, 59, 15987–15991. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Correia, J.H. Enhanced photodynamic therapy: A review of combined energy sources. Cells 2022, 11, 3995. [Google Scholar] [CrossRef]
- Shen, J.C.; He, W. The fabrication strategies of near-infrared absorbing transition metal complexes. Coord. Chem. Rev. 2023, 483, 215096. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly phosphorescent bis-cyclometalated iridium complexes: Synthesis, photophysical characterization, and use in organic light emitting diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Yoon, S.; Teets, T.S. Enhanced deep red to near-infrared(DR-NIR) phosphorescence in cyclometalated iridium(III) complexes. Inorg. Chem. Front. 2022, 9, 6544–6553. [Google Scholar] [CrossRef]
- Di Censo, D.; Fantacci, S.; De Angelis, F.; Klein, C.; Evans, N.; Kalyanasundaram, K.; Bolink, H.J.; Gratzel, M.; Nazeeruddin, M.K. Synthesis, characterization, and DFT/TD-DFT calculations of highly phosphorescent blue light-emitting anionic iridium complexes. Inorg. Chem. 2008, 47, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F.; Yuskevitch, Y.; Wantz, G.; Mayer, C.R.; Bertin, D.; Gigmes, D. Light-emitting electrochemical cells based on a solution-processed multilayered device and an anionic iridium(III) complex. Synth. Met. 2013, 177, 100–104. [Google Scholar] [CrossRef]
- Ceron-Carrasco, J.P.; Zuniga, J.; Requena, A. Tuning the optical properties of novel antitumoral drugs based on cyclometalated iridium(III) complexes. J. Phys. Chem. A 2019, 123, 8644–8649. [Google Scholar] [CrossRef] [PubMed]
- Tatarin, S.V.; Kalle, P.; Taydakov, I.V.; Varaksina, E.A.; Korshunov, V.M.; Bezzubov, S.I. Sterically hindered phenanthroimidazole ligands drive the structural flexibility and facile ligand exchange in cyclometalated iridium(III) complexes. Dalton Trans. 2021, 50, 6889–6900. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.Y.; Wang, Y.S.; Hou, T.J.; Shen, X. Novel deep red to near-infrared phosphorescent iridium(III) complexes bearing pyrenyl: Syntheses, structures and modulation of the photophysical properties. Inorg. Chem. Commun. 2023, 150, 110460. [Google Scholar] [CrossRef]
- Ho, P.Y.; Lee, S.Y.; Kam, C.; Zhu, J.F.; Shan, G.G.; Hong, Y.N.; Wong, W.Y.; Chen, S.J. Fluorescence imaging and photodynamic inactivation of bacteria based on cationic cyclometalated iridium(III) complexes with aggregation-induced emission properties. Adv. Healthc. Mater. 2021, 10, 2100706. [Google Scholar] [CrossRef] [PubMed]
- Stonelake, T.M.; Phillips, K.A.; Otaif, H.Y.; Edwardson, Z.C.; Horton, P.N.; Coles, S.J.; Beames, J.M.; Pope, S.J.A. Spectroscopic and theoretical investigation of color tuning in deep-red luminescent iridium(III) complexes. Inorg. Chem. 2020, 59, 2266–2277. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.M.; Cui, P.; Hetu, M.; Wang, C.Z.; Monro, S.; Schaller, R.D.; Cameron, C.G.; Liu, B.Q.; Kilina, S.; et al. Near-infrared-emitting heteroleptic cationic iridium complexes derived from 2,3-diphenylbenzo[g] quinoxaline as in vitro theranostic photodynamic therapy agents. Dalton Trans. 2017, 46, 8091–8103. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Ma, D.L.; Wong, S.Y.; Kang, T.S.; Ng, H.P.; Han, Q.B.; Leung, C.H. Iridium(III)-based chemosensors for the detection of metal ions. Methods 2019, 168, 3–17. [Google Scholar] [CrossRef]
- Lin, C.K.; Wang, J.; Yang, K.; Liu, J.B.; Ma, D.L.; Leung, C.H.; Wang, W.H. Development of a NIR iridium(III) complex for self-calibrated and luminogenic detection of boron trifluoride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121658. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Liu, J.H.; Kong, L.T.; Wang, L.; Niu, D.; Wang, J.; Leung, C.H. Synthesis and luminescence monitoring of iridium(III) complex-functionalized gold nanoparticles and their application for determination of gold(III) ions. Mikrochim. Acta 2023, 190, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Wang, X.L.; Wang, J.; Leung, C.H.; Wang, W.H. Imaging mitochondrial palladium species in living cells with a NIR iridium(III) complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 288, 122188. [Google Scholar] [CrossRef] [PubMed]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef]
- Xia, Q.F.; Feng, S.M.; Liu, D.D.; Feng, G.Q. A highly selective and sensitive colorimetric and near-infrared fluorescent turn-on probe for rapid detection of palladium in drugs and living cells. Sens. Actuators B Chem. 2018, 258, 98–104. [Google Scholar] [CrossRef]
- Dolai, B.; Nayim, S.; Hossain, M.; Pahari, P.; Atta, A.K. A triazole linked C-glycosyl pyrene fluorescent sensor for selective detection of Au3+ in aqueous solution and its application in bioimaging. Sens. Actuators B Chem. 2019, 279, 476–482. [Google Scholar] [CrossRef]
- Nam, S.H.; Lee, W.M.; Shin, Y.J.; Yoon, S.J.; Kim, S.W.; Kwak, J.I.; An, Y.J. Derivation of guideline values for gold(III) ion toxicity limits to protect aquatic ecosystems. Water Res. 2014, 48, 126–136. [Google Scholar] [CrossRef]
- Lu, N.; Luo, Y.H.; Zhang, Q.L.; Zhang, P.Y. Microenvironment-sensitive iridium(III) complexes for disease theranostics. Dalton Trans. 2020, 49, 9182–9190. [Google Scholar] [CrossRef]
- Lee, L.C.C.; Lo, K.K.W. Strategic design of luminescent rhenium(I), ruthenium(II), and iridium(III) complexes as activity-based probes for bioimaging and biosensing. Chem. Asian. J. 2022, 29, e202200840. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zhang, G.X.; Zhao, R.; Zhang, D.Q. Tetraphenylethene-based cis/trans isomers for targeted fluorescence sensing and biomedical applications. Chem. Eur. J. 2023, 29, e202300539. [Google Scholar] [CrossRef]
- Shi, H.D.; Wang, Y.; Lin, S.M.; Lou, J.X.; Zhang, Q.L. Recent development and application of cyclometalated iridium(III) complexes as chemical and biological probes. Dalton Trans. 2021, 50, 6410–6417. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.W.; Li, S.P.Y.; Zhang, K.Y. Development of luminescent iridium(III) polypyridine complexes as chemical and biological probes. New J. Chem. 2011, 35, 265–287. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef]
- Zhan, Z.X.; Su, Z.S.; Chai, L.; Li, C.H.; Liu, R.; Lv, Y. Multimodal imaging iridium(III) complex for hypochlorous acid in living systems. Anal. Chem. 2020, 92, 8285–8291. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.C.; Zhan, Z.X.; Chai, L.; Zhang, L.C.; Guo, Q.; Zhang, K.X.; Lv, Y. A two-photon excited near-infrared iridium(III) complex for multi-signal detection and multimodal imaging of hypochlorite. Anal. Chem. 2021, 93, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, K.; Yuan, X.; Xie, X.; Zhan, Z.; Lv, Y. Novel near-infrared iridium(III) complex for chemiluminescence imaging of hypochlorous acid. Anal. Chem. 2023, 95, 8310–8317. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Chen, J.; Xu, Z.C. Fluorescent probes for biothiols based on metal complex. Coord. Chem. Rev. 2021, 429, 213638. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Wu, R.M.; Li, H.F.; Zeng, H.; Li, Y.Y.; Wang, Q.H.; Shi, M.; Fan, X.L. Near-infrared phosphorescent iridium(III) complex for imaging of cysteine and homocysteine in living cells and in vivo. RSC Adv. 2017, 7, 52621–52625. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wu, Y.Q.; Wu, J.; Lun, W.C.; Zeng, H.; Fan, X.L. A near-infrared phosphorescent iridium(III) complex for fast and time-resolved detection of cysteine and homocysteine. Analyst 2020, 145, 2238–2244. [Google Scholar] [CrossRef]
- Huang, L.L.; Leung, P.K.K.; Lee, L.C.C.; Xu, G.X.; Lam, Y.W.; Lo, K.K.W. Photofunctional cyclometallated iridium(III) polypyridine methylsulfone complexes as sulfhydryl-specific reagents for bioconjugation, bioimaging and photocytotoxic applications. Chem. Commun. 2022, 58, 10162–10165. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.J.; Zhao, C.; Zhang, H.R.; Zhu, M.Z.; Xu, Q.; Wang, X.Q.; Zhao, C.M.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wu, Y.Q.; Chen, L.Y.; Zeng, H.; Chen, X.Y.; Lun, W.C.; Fan, X.L.; Wong, W.Y. A time-resolved near-infrared phosphorescent iridium(III) complex for fast and highly specific peroxynitrite detection and bioimaging applications. J. Mater. Chem. B 2019, 7, 7612–7618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Meng, X.C.; Zou, L.; Zhao, M.L.; Liu, S.J.; Tao, P.; Jiang, J.Y.; Zhao, Q. A dual-emissive phosphorescent polymeric probe for exploring drug-induced liver injury via imaging of peroxynitrite elevation in vivo. ACS Appl. Mater. Interfaces 2020, 12, 12383–12394. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liao, X.; Chen, Y.; Ji, L.; Chao, H. Mitochondria-targeting and reversible near-infrared emissive iridium(III) probe for in vivo ONOO−/GSH redox cycles monitoring. Anal. Chem. 2021, 93, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Wen, J.; Chen, S.S.; Sun, S.G. A luminescent bimetallic iridium(III) complex for ratiometric tracking intracellular viscosity. Chem. Commun. 2018, 54, 4061. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, I.S.; Elistratova, A.A.; Sokolov, V.V.; Chelushkin, P.S.; Shirmanova, M.V.; Lukina, M.M.; Dudenkova, V.V.; Shcheslayskiy, V.I.; Kalinina, S.; Reess, K.; et al. A biocompatible phosphorescent Ir(III) oxygen sensor functionalized with oligo(ethylene glycol) groups: Synthesis, photophysics and application in PLIM experiments. New J. Chem. 2020, 44, 10459–10471. [Google Scholar] [CrossRef]
- Kritchenkov, I.S.; Solomatina, A.I.; Kozina, D.O.; Porsev, V.V.; Sokolov, V.V.; Shirmanova, M.V.; Lukina, M.M.; Komarova, A.D.; Shcheslayskiy, V.I.; Belyaeva, T.N.; et al. Biocompatible Ir(III) complexes as oxygen sensors for phosphorescence lifetime imaging. Molecules 2021, 26, 2898. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Muraoka, T.; Shiozaki, S.; Tobita, S.; Yoshihara, T. Near-infrared emitting Ir(III) complexes bearing a dipyrromethene ligand for oxygen imaging of deeper tissues in vivo. Anal. Chem. 2022, 94, 2794–2802. [Google Scholar] [CrossRef]
- Yu, H.C.; Yu, B.; Song, Y.J.; Hai, P. Recent advances of cyclometalated Ir(III) complexes for optical oxygen sensing. Inorg. Chim. Acta 2023, 550, 121435. [Google Scholar] [CrossRef]
- Kritchenkov, I.S.; Mikhnevich, V.G.; Stashchak, V.S.; Solomatina, A.I.; Kozina, D.O.; Sokolov, V.V.; Tunik, S.P. Novel NIR-Phosphorescent Ir(III) complexes: Synthesis, characterization and their exploration as lifetime-based O2 sensors in living cells. Molecules 2022, 27, 3156. [Google Scholar] [CrossRef]
- Zamora, A.; Vigueras, G.; Rodriguez, V.; Santana, M.D.; Ruiz, J. Cyclometalated iridium(III) luminescent complexes in therapy and phototherapy. Coord. Chem. Rev. 2018, 360, 34–76. [Google Scholar] [CrossRef]
- Tao, P.; Lv, Z.; Zheng, X.K.; Jiang, H.; Liu, S.J.; Wang, H.; Wong, W.Y.; Zhao, Q. Isomer engineering of lepidine-based iridophosphors for far-red hypoxia imaging and photodynamic therapy. Inorg. Chem. 2022, 61, 17703–17712. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Y.; Liang, B.F.; Zhi, Y.S.; Yao, D.H.; Li, C.Y.; Wu, H.Q.; He, L. Near-infrared AIE-active phosphorescent iridium(III) complex for mitochondria-targeted photodynamic therapy. Dalton Trans. 2023, 52, 1291–1300. [Google Scholar] [CrossRef]
- Yuan, H.; Han, Z.; Chen, Y.C.; Qi, F.; Fang, H.B.; Guo, Z.J.; Zhang, S.R.; He, W.J. Ferroptosis photoinduced by new cyclometalated iridium(III) complexes and its synergism with apoptosis in tumor cell inhibition. Angew. Chem. Int. Ed. 2021, 60, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Chen, F.; Song, Y.-Q.; Nao, S.-C.; Chan, D.S.-H.; Wong, C.-Y.; Wang, W.; Leung, C.-H. A glycyrrhetinic acid-iridium(III) conjugate as a theranostic NIR probe for hepatocellular carcinoma with mitochondrial-targeting ability. Eur. J. Med. Chem. 2024, 264, 115995. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Yuan, X.L.; Li, S.F.; Le Guennic, B.; Ma, J.; Zhang, C.S.; Jacquemin, D.; Zhao, J.Z. Cyclometalated Ir(III) complexes with styryl-BODIPY ligands showing near IR absorption/emission: Preparation, study of photophysical properties and application as photodynamic/luminescence imaging materials. J. Mater. Chem. B 2014, 2, 2838–2854. [Google Scholar] [CrossRef]
- Zhang, L.P.; Geng, Y.; Li, L.J.; Tong, X.F.; Liu, S.; Liu, X.M.; Su, Z.M.; Xie, Z.G.; Zhu, D.X.; Bryce, M.R. Rational design of iridium-porphyrin conjugates for novel synergistic photodynamic and photothermal therapy anticancer agents. Chem. Sci. 2021, 12, 5918–5925. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Wu, J.; Wong, W.Y. A new near-infrared phosphorescent iridium(III) complex conjugated to a xanthene dye for mitochondria-targeted photodynamic therapy. Biomater. Sci. 2021, 9, 4843–4853. [Google Scholar] [CrossRef]
- Novohradsky, V.; Rovira, A.; Hally, C.; Galindo, A.; Vigueras, G.; Gandioso, A.; Svitelova, M.; Bresoli-Obach, R.; Kostrhunova, H.; Markova, L.; et al. Towards novel photodynamic anticancer agents generating superoxide anion radicals: A cyclometalated Ir(III) complex conjugated to a far-red emitting coumarin. Angew. Chem. Int. Ed. 2019, 58, 6311–6315. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, H.Y.; Gao, Y.C.; Lin, J.M.; Yang, H.; Yang, S.P. Photostable iridium(III)-cyanine complex nanoparticles for photoacoustic imaging guided near-infrared photodynamic therapy in vivo. ACS Appl. Mater. Interfaces 2019, 11, 15417–15425. [Google Scholar] [CrossRef]
- Wu, W.B.; Mao, D.; Hu, F.; Xu, S.D.; Chen, C.; Zhang, C.J.; Cheng, X.M.; Yuan, Y.Y.; Ding, D.; Kong, D.L.; et al. A highly efficient and photostable photosensitizer with near-infrared aggregation-induced emission for image-guided photodynamic anticancer therapy. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.H.; Zhao, S.J.; Liu, W.M.; Lee, C.S.; Zhang, W.J.; Wang, P.F. Photosensitizers for photodynamic therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yu, B.; Han, G.Y.; Liu, M.H.; Shan, B.; Dong, G.Q.; Miao, Z.Y.; Jia, N.Y.; Tan, Z.; Li, B.H.; et al. Chlorin p6-based water-soluble amino acid derivatives as potent photosensitizers for photodynamic therapy. J. Med. Chem. 2016, 59, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.C.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [PubMed]
- Sgambellone, M.A.; David, A.; Garner, R.N.; Dunbar, K.R.; Turro, C. Cellular toxicity induced by the photorelease of a caged bioactive molecule: Design of a potential dual-action Ru(II) complex. J. Am. Chem. Soc. 2013, 135, 11274–11282. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, N.E.; Tanrıverdi Eçik, E.; Kazan, H.H.; Yenilmez Çiftçi, G.; Yuksel, F. Novel ruthenium(II) and iridium(III) BODIPY dyes: Insights into their application in photodynamic therapy in vitro. Photochem. Photobiol. Sci. 2019, 18, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Li, L.J.; Wang, W.J.; Wang, R.L.; Li, G.Z.; Bian, H.; Zhu, D.X.; Bryce, M.R. Self-assembled nanoparticles based on cationic mono-/AIE tetra-nuclear Ir(III) complexes: Long wavelength absorption/near-infrared emission photosensitizers for photodynamic therapy. Dalton Trans. 2023, 52, 1595–1601. [Google Scholar] [CrossRef]
- Zhou, L.H.; Wei, F.F.; Xiang, J.J.; Li, H.F.; Li, C.B.; Zhang, P.F.; Liu, C.J.; Gong, P.; Cai, L.T.; Wong, K.M.C. Enhancing the ROS generation ability of a rhodamine-decorated iridium(III) complex by ligand regulation for endoplasmic reticulum-targeted photodynamic therapy. Chem. Sci. 2020, 11, 12212–12220. [Google Scholar] [CrossRef]
- Liu, Z.W.; Song, F.L.; Shi, W.B.; Gurzadyan, G.; Yin, H.Y.; Song, B.; Liang, R.; Peng, X.J. Nitroreductase-activatable theranostic molecules with high PDT efficiency under mild hypoxia based on a TADF fluorescein derivative. ACS Appl. Mater. Interfaces 2019, 11, 15426–15435. [Google Scholar] [CrossRef]
- Thambi, T.; Park, J.H.; Lee, D.S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: Recent approaches and future perspectives. Chem. Commun. 2016, 52, 8492–8500. [Google Scholar] [CrossRef]
- Liu, J.N.; Bu, W.B.; Shi, J.L. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef] [PubMed]
- Bevernaegie, R.; Doix, B.; Bastien, E.; Diman, A.; Decottignies, A.; Feron, O.; Elias, B. Exploring the phototoxicity of hypoxic active iridium(III)-based sensitizers in 3D tumor spheroids. J. Am. Chem. Soc. 2019, 141, 18486–18491. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Ortega-Forte, E.; Hally, C.; Jorda-Redondo, M.; Abad-Montero, D.; Vigueras, G.; Martinez, J.I.; Bosch, M.; Nonell, S.; Ruiz, J.; et al. Exploring structure-activity relationships in photodynamic therapy anticancer agents based on Ir(III)-COUPY conjugates. J. Med. Chem. 2023, 66, 7849–7867. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Xu, Q.; Wang, W.J.; Shao, J.J.; Huang, W.; Dong, X.C. Type I photosensitizers revitalizing photodynamic oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Y.; Wang, Y.; Wang, J.; Lin, J.; Du, J.; Zhou, Z.; Liu, X.; Yang, S.; Yang, H. Bifunctional Nano-Assembly of Iridium(III) Phthalocyanine Complex Encapsulated with BSA: Hypoxia-relieving/Sonosensitizing Effects and their Immunogenic Sonodynamic Therapy. Adv. Funct. Mater. 2023, 33, 2210348. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. Energy gap law for radlatIonless transitions in large molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, K.W.; Xu, G.; Liu, X.; Zhao, Q.; Xu, C.J.; Gou, S.H. An iridium(III) complex bearing a donor-acceptor-donor type ligand for NIR-triggered dual phototherapy. Adv. Funct. Mater. 2021, 31, 2008325. [Google Scholar] [CrossRef]
- Wang, W.; Vellaisamy, K.; Li, G.; Wu, C.; Ko, C.-N.; Leung, C.-H.; Ma, D.-L. Development of a long-lived luminescence probe for visualizing β-galactosidase in ovarian carcinoma cells. Anal. Chem. 2017, 89, 11679–11684. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Lin, S.; Vellaisamy, K.; Li, G.; Tan, W.; Leung, C.-H.; Ma, D.-L. First synthesis of an oridonin-conjugated iridium(III) complex for the intracellular tracking of NF-κB in living cells. Chem. Eur. J. 2017, 23, 4929–4935. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yang, K.; Hu, S.; Wang, W. Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications. Biosensors 2022, 12, 683. [Google Scholar] [CrossRef]


















| Direct Modification with Cyclometalated Ligand | Indirect Modification with NIR Dye | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Complexes | Target | Solvent | λabs/nm | λemi/nm | ΦPL | Lifetime/ns | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | BF3 | ACN | 280 (DMF) | 475, 650 | 0.24 | 356.0 | [51] |
| 2 | Pd species | ACN | 260 | 670 | 0.0641 | 314.8 | [53] |
| 3 | Au3+ | ACN | 365 | 700 | 0.0606 | 368.8 | [52] |
| Complexes | Target | Solvent | λabs/nm | λemi/nm | ΦPL | Lifetime/ns | Ref. |
|---|---|---|---|---|---|---|---|
| 4a | HClO | DMF/PBS | 318, 408 | / | 0.00002 | 3.78 | [64] |
| 4b | / | DMF/PBS | 310, 408 | 540, 570 | 0.00178 | 369.1 | [64] |
| 5a | ClO− | MeOH/PBS | 284, 398, 502 | 663 | 0.002 | 165 | [65] |
| 5b | / | MeOH/PBS | 285, 361,498 | 662 | 0.104 | 382 | [65] |
| 6a | HClO | DMF/PBS | 370, 472 | 663 | 0.00076 | / | [66] |
| 6b | / | DMF/PBS | 369, 470 | 663 | 0.00052 | / | [66] |
| 7a | Cys/Hcy | EtOH | 261, 345,500 | 680 | 0.008 | 158 | [68] |
| 7b | / | EtOH | 261, 345, 500 | 670 | 0.018 | / | [68] |
| 7c | / | EtOH | 261, 345, 500 | 670 | 0.021 | / | [68] |
| 8a | Cys/Hcy | DMSO/PBS | 486, 303 | 683, 748 | 0.005 | / | [69] |
| 8b | / | DMSO/PBS | 286, 310, 358, 486 | 683, 748 | 0.109 | / | [69] |
| 8c | / | DMSO/PBS | 285, 311, 358, 486 | 683, 748 | 0.122 | / | [69] |
| 9 | Cys-bearing peptides and proteins | ACN | / | 668 | 0.048 | 346 | [70] |
| 10a | ONOO− | DMSO/PBS | 289, 320, 360, 500 | 660, 710 | 0.012 | 7490 | [72] |
| 10b | / | DMSO/PBS | 289, 324, 350, 500 | 660, 710 | 0.131 | 7140 | [72] |
| 11 | ONOO− | H2O | / | 605, 678/743 | 0.04 | 600/680 | [73] |
| 12 | ONOO−/GSH | DMSO/PBS | 302 | 704 | 0.136 | / | [74] |
| Complexes | Solvent | λabs/nm | λemi/nm | ΦPL | Lifetime/ns | Ref. |
|---|---|---|---|---|---|---|
| 13 | ACN | / | 521, 708 | 0.063 (H2O) | 1770 | [75] |
| 14a | MeOH | 331, 373, 393, 424, 496, 524 | 710, 775, 881, 943 | 0.014 | 270 | [76] |
| 14b | MeOH | 336, 374, 390, 433, 511, 534 | 719, 782, 882, 941 | 0.014 | 280 | [76] |
| 14c | MeOH | 336, 373, 392, 433, 511, 534s | 720, 782, 882, 940 | 0.015 | 290 | [76] |
| 15a | H2O | 252, 306, 344, 370, 441, 530, 570 | 728, 790, 900 | 0.084 | 2340 | [77] |
| 15b | H2O | 253, 306, 345, 369, 441, 532, 570 | 727, 789, 900 | 0.082 | 2160 | [77] |
| 16a | MeOH | 263, 298, 338, 367, 430, 475, 505, 525 | 717, 784, 885 | 0.017 | 410 | [80] |
| 16b | MeOH | 263, 307, 344, 380, 430, 445, 525, 541 | 720, 783, 890 | 0.023 | 580 | [80] |
| Complexes | Solvent | λabs/nm | λemi/nm | ΦPL | ΦΔ | Lifetime/ns | Ref. |
|---|---|---|---|---|---|---|---|
| 17 | CH2Cl2 | 331,385,413, 437, 490, 540, 756 | 800, 915, 970 | 0.0017 | 0.56 (ACN) | 360 | [48] |
| 18 | CH2Cl2 | 275,352,537 | 650 | 0.35 | 0.73 (MeOH) | 1940 | [82] |
| 19 | ACN | / | 650–750 | 0.058 | / | / | [83] |
| 20a | PBS | 488 | 685 | 0.004 | 0.81 | / | [84] |
| 20b | PBS | 488 | 685 | 0.005 | 0.76 | / | [84] |
| 21 | PBS | 370 | 686 | 0.197 | / | 1520 | [85] |
| Complexes | Solvent | λabs/nm | λemi/nm | ΦPL | ΦΔ | Lifetime/ns | Ref. |
|---|---|---|---|---|---|---|---|
| 22a | Toluene | 563, 606 | 624 | 0.399 | 0.53 (CH2Cl2) | 106,600 | [86] |
| 22b | Toluene | 610, 664 | 621, 691 | 0.132 | 0.81 (CH2Cl2) | 156,500 | [86] |
| 22c | Toluene | 596, 644 | 683 | 0.326 | 0.06 (CH2Cl2) | 92,500 | [86] |
| 22d | Toluene | 268, 729 | 684, 794 | 0.01 | 0.02 (CH2Cl2) | 31,400 | [86] |
| 23 | DMSO | 352, 577, 629 | 642 | / | 0.06 | 4.70 | [96] |
| 24a | H2O | 420; 518 | 657, 720 | 0.11 | 0.72 | 4.67 | [87] |
| 24b | H2O | 427; 519 | 660, 724 | 0.05 | 0.89 | 4.82 | [87] |
| 25a | H2O | 257, 417, 520, 558, 594, 650 | 656, 720 | 0.081 | / | 5.72 | [97] |
| 25b | H2O | 256, 424, 521, 560, 594, 650 | 656, 720 | 0.036 | / | 5.87 | [97] |
| 26a | EtOH | 293, 356, 510 | 652, 704 | 0.030 | 0.72 (PBS) | 603 | [88] |
| 26b | EtOH | 305, 356, 508 | 651, 705 | 0.018 | 0.29 (PBS) | 408 | [88] |
| 27a | PBS | 305 | 656 | >0.01 | <0.01 | 55 (93%) 281 (7%) | [89] |
| 27b | PBS | 550 | 615 | 0.004 | <0.01 | 0.37 (73%) 3.3 (27%) | [89] |
| 28a | H2O | 685 | / | <0.01 | 0.31 | 9.78 | [32] |
| 28b | H2O | 678 | / | <0.01 | 0.53 | / | [32] |
| 29 | MeOH/H2O | 814 | 1050 | 0.0017 | 0.146 | / | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, S.; Wu, X.; Niu, D.; Wang, J.; Leung, C.-H.; Wang, W. Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy. Molecules 2024, 29, 256. https://doi.org/10.3390/molecules29010256
Jing S, Wu X, Niu D, Wang J, Leung C-H, Wang W. Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy. Molecules. 2024; 29(1):256. https://doi.org/10.3390/molecules29010256
Chicago/Turabian StyleJing, Shaozhen, Xiaolei Wu, Dou Niu, Jing Wang, Chung-Hang Leung, and Wanhe Wang. 2024. "Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy" Molecules 29, no. 1: 256. https://doi.org/10.3390/molecules29010256
APA StyleJing, S., Wu, X., Niu, D., Wang, J., Leung, C. -H., & Wang, W. (2024). Recent Advances in Organometallic NIR Iridium(III) Complexes for Detection and Therapy. Molecules, 29(1), 256. https://doi.org/10.3390/molecules29010256