Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment
Abstract
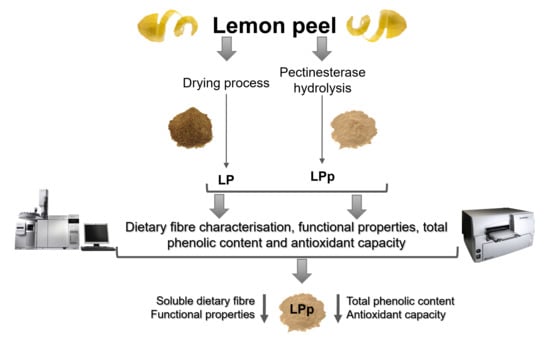
:1. Introduction
2. Results and Discussion
2.1. Dietary Fibre Content
2.2. Dietary Fibre Characterisation by GC-FID
2.3. Functional Properties
2.4. Total Phenolic Content
2.5. Antioxidant Capacity
3. Materials and Methods
3.1. Samples
3.2. Enzymatic Treatment
3.3. Dietary Fibre Quantification
3.4. Dietary Fibre Characterisation through Gas Chromatography
3.5. Functional Properties
3.6. (Poly)phenol Extraction and Total Phenolic Content Analysis
3.7. Antioxidant Capacity Analysis
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Del Rio Osorio, L.L.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Xu, Y.; Chen, L.; Cao, J.; Jiang, W. An Advance on Nutritional Profile, Phytochemical Profile, Nutraceutical Properties, and Potential Industrial Applications of Lemon Peels: A Comprehensive Review. Trends Food Sci. Technol. 2022, 124, 219–236. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Azman, N.F.I.N.; Azlan, A.; Khoo, H.E.; Razman, M.R. Antioxidant Properties of Fresh and Frozen Peels of Citrus Species. Curr. Res. Nutr. Food Sci. 2019, 7, 331–339. [Google Scholar] [CrossRef]
- Uçak, İ.; Khalily, R. Effects of Different Solvent Extractions on the Total Phenolic Content and Antioxidant Activity of Lemon and Orange Peels. Eurasian J. Food Sci. Technol. 2022, 6, 23–28. [Google Scholar]
- Kaya, M.; Sousa, A.G.; Crépeau, M.J.; Sørensen, S.O.; Ralet, M.C. Characterization of Citrus Pectin Samples Extracted under Different Conditions: Influence of Acid Type and PH of Extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Dietary Fiber Extraction for Human Nutrition—A Review. Food Rev. Int. 2016, 32, 98–115. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Interaction of Phenolics and Their Association with Dietary Fiber. In Dietary Fiber Functionality in Food and Nutraceuticals: From Plant to Gut; Wiley: Hoboken, NJ, USA, 2017; pp. 21–44. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-Extractable Polyphenols, a Major Dietary Antioxidant: Occurrence, Metabolic Fate and Health Effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Carboni Martins, C.; Rodrigues, R.C.; Domeneghini Mercali, G.; Rodrigues, E. New Insights into Non-Extractable Phenolic Compounds Analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). FoodData Central: Lemon Peel Composition. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/167749/nutrients (accessed on 27 September 2023).
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-Products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical Characterization of Five Types of Citrus Dietary Fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Qi, B.; Jiang, L.; Li, Y.; Chen, S.; Sui, X. Extract Dietary Fiber from the Soy Pods by Chemistry-Enzymatic Methods. Procedia Eng. 2011, 15, 4862–4873. [Google Scholar] [CrossRef]
- Fuso, A.; Viscusi, P.; Larocca, S.; Sangari, F.S.; Lolli, V.; Caligiani, A. Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective. Foods 2023, 12, 148. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Benito, M.J.; Martín, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Casquete, R. Improve the Functional Properties of Dietary Fibre Isolated from Broccoli By-Products by Using Different Technologies. Innov. Food Sci. Emerg. Technol. 2022, 80, 103075. [Google Scholar] [CrossRef]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the Water- and Alkali-Soluble Hemicelluloses Fractionated by Sequential Acidification and Graded-Ethanol from Sweet Maize Stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef]
- Mendez, D.A.; Fabra, M.J.; Martínez-Abad, A.; Μartínez-Sanz, M.; Gorria, M.; López-Rubio, A. Understanding the Different Emulsification Mechanisms of Pectin: Comparison between Watermelon Rind and Two Commercial Pectin Sources. Food Hydrocoll. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Samsudin, D.; Gan, C.Y. Averrhoa Bilimbi Pectin-Based Edible Films: Effects of the Linearity and Branching of the Pectin on the Physicochemical, Mechanical, and Barrier Properties of the Films. Int. J. Biol. Macromol. 2020, 163, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Samuel, P. Fiber Ingredients: Food Applications and Health Benefits; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420043853. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic Compounds in Agri-Food by-Products, Their Bioavailability and Health Effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Qi, J. Functional and Structural Properties of Dietary Fiber from Citrus Peel Affected by the Alkali Combined with High-Speed Homogenization Treatment. LWT 2020, 128, 109397. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. [Google Scholar] [CrossRef]
- Pieracci, Y.; Pistelli, L.; Cecchi, M.; Pistelli, L.; De Leo, M. Phytochemical Characterization of Citrus-Based Products Supporting Their Antioxidant Effect and Sensory Quality. Foods 2022, 11, 1550. [Google Scholar] [CrossRef]
- Shu, B.; Wu, G.; Wang, Z.; Wang, J.; Huang, F.; Dong, L.; Zhang, R.; Wang, Y.; Su, D. The Effect of Microwave Vacuum Drying Process on Citrus: Drying Kinetics, Physicochemical Composition and Antioxidant Activity of Dried Citrus (Citrus reticulata Blanco) Peel. J. Food Meas. Charact. 2020, 14, 2443–2452. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R. Antioxidant Activity of Water-Soluble Maillard Reaction Products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of Insoluble, Soluble, and Total Dietary Fiber in Foods and Food Products: Interlaboratory Study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Rockville, MD, USA, 2016; Volume II. [Google Scholar]
- Englyst, H.N.; Quigley, M.E.; Hudson, G.J.; Cummings, J.H. Determination of Dietary Fibre as Non-Starch Polysaccharides by Gas-Liquid Chromatography. Analyst 1992, 117, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W. Colorimetric Determination of Hexuronic Acids in Plant Materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Umaña, M.; Eim-Iznardo, V.; Roselló, M. Cinéticas de Extracción y Caracterización de Pectinas de Los Subproductos de Naranja Mediante Asistencia Acústica. Master’s Thesis, Universitat de Les Illes Balears, Palma, Spain, 2016. [Google Scholar]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative Study of the Cell Wall Composition of Broccoli, Carrot, and Tomato: Structural Characterization of the Extractable Pectins and Hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 114–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]




| Composition (%) | LP | LPp |
|---|---|---|
| Rhamnose | 3.3 ± 0.2 | 24.1 ± 1.2 * |
| Fucose | 1.1 ± 0.3 * | 0.2 ± 0.2 |
| Arabinose | 31.6 ± 1.4 * | 9.3 ± 0.9 |
| Xylose | 9.4 ± 1.3 * | 0.8 ± 0.2 |
| Mannose | 7.3 ± 0.3 * | 4.4 ± 0.3 |
| Galactose | 21.4 ± 0.6 | 25.5 ± 0.8 * |
| Glucose | 9.1 ± 0.2 | 20.8 ± 1.2 * |
| Uronic acids | 16.9 ± 0.1 * | 14.9 ± 0.1 |
| Cellulose 1 | 8.2 ± 0.2 | 18.7 ± 1.0 * |
| Hemicellulose 2 | 18.7 ± 1.2 | 7.5 ± 0.4 * |
| Pectin 3 | 73.1 ± 1.1 | 73.8 ± 0.7 |
| Parameter | LP | LPp |
|---|---|---|
| Mannans to hemicelluloses contr 1 | 0.8 ± 0.1 | 5.7 ± 1.6 * |
| Linearity of pectin 2 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Rhamnose and uronic acid contr 3 | 0.2 ± 0.0 | 1.6 ± 0.1 * |
| RG-I Branching 4 | 16.1 ± 1.3 * | 1.5 ± 0.1 |
| Functional Property | LP | LPp |
|---|---|---|
| SWC (mL water/g) | 9.9 ± 0.07 *,1 | 2.6 ± 0.0 |
| WRC (g water/g) | 1.1 ± 0.0 * | 0.8 ± 0.1 |
| FAC (g oil/g) | 9.3 ± 0.6 * | 2.7 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Gómez, V.; San Mateo, M.; González-Barrio, R.; Periago, M.J. Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment. Molecules 2024, 29, 269. https://doi.org/10.3390/molecules29010269
Núñez-Gómez V, San Mateo M, González-Barrio R, Periago MJ. Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment. Molecules. 2024; 29(1):269. https://doi.org/10.3390/molecules29010269
Chicago/Turabian StyleNúñez-Gómez, Vanesa, Marta San Mateo, Rocío González-Barrio, and Mª Jesús Periago. 2024. "Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment" Molecules 29, no. 1: 269. https://doi.org/10.3390/molecules29010269
APA StyleNúñez-Gómez, V., San Mateo, M., González-Barrio, R., & Periago, M. J. (2024). Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment. Molecules, 29(1), 269. https://doi.org/10.3390/molecules29010269