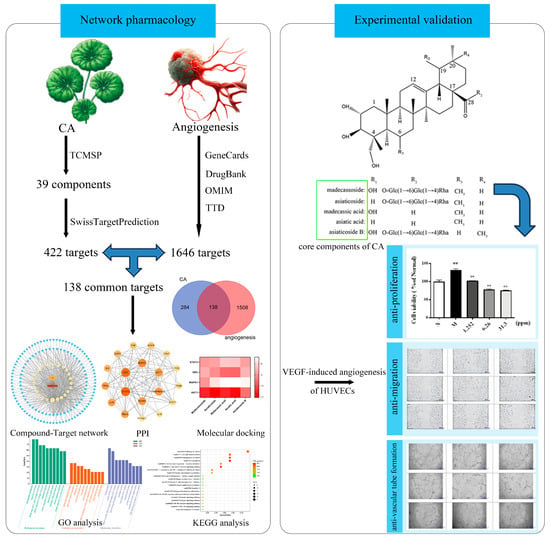
Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation
Abstract
:1. Introduction
2. Results
2.1. Network Pharmacology
2.1.1. Targets of CA and Angiogenesis
2.1.2. Common Target Acquisition and PPI Network Construction
2.1.3. Core Targets Analysis
2.1.4. GO and KEGG Enrichment Analyses
2.1.5. Molecular Docking Analysis
2.2. Experimental Outcomes
2.2.1. Cellular Anti-Proliferation Activity
2.2.2. The Cellular Anti-Migration Activity of Components from CA
2.2.3. The Cellular Anti-Vascular Tube Formation Activity
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology
4.1.1. Identification of Active Components and Potential Targets of CA
4.1.2. Screening of Potential Target Genes for Angiogenesis
4.1.3. Intersection of Targets for CA and Angiogenesis-Related Targets
4.1.4. Construction of the Protein–Protein Interaction (PPI) Network
4.1.5. Determination of Core Targets
4.1.6. GO and KEGG Enrichment Analyses
4.1.7. Molecular Docking
4.2. In Vitro Experiments
4.2.1. Materials
4.2.2. Cell Culture and Drug Treatment
4.2.3. Cell Proliferation Assay
4.2.4. Cell Migration Assay
4.2.5. Vascular Tube Formation Assay
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Centella asiatica |
| TCM | traditional Chinese medicine |
| TCMSP | traditional Chinese medicine systems pharmacology |
| PPI | protein–protein interaction |
| GO | gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| VEGF | vascular endothelial growth factor |
| STAT3 | signal transducer and activator of transcription 3 |
| MAPK1 | mitogen-activated protein kinase 1 |
| PD-1 | programmed cell death protein 1 |
| HUVECs | human umbilical vein endothelial cells |
| CTX | cyclophosphamide |
| BP | biological process |
| MF | molecular function |
| CC | cellular component |
| DL | drug-like |
| FBS | fetal bovine serum |
| FAK | focal adhesion kinase |
| EC | endometrial carcinoma |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal. Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Jászai, J.; Schmidt, M.H.H. Trends and challenges in tumor anti-angiogenic therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Q.; Shao, Y.; Yin, S.; Liu, C.; Liu, Y.; Wang, R.; Wang, T.; Qiu, Y.; Yu, H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 2021, 133, 111044. [Google Scholar] [CrossRef]
- Xia, B.; Li, Y.; Liu, Y.; Sun, W.; Chen, J.; Li, L.; Pang, J.; Liu, X.; Chen, S.; Cheng, H. Rapid separation of asiatic acid, quercetin, and kaempferol from traditional chinese medicine Centella asiatica (L.) urban using HSCCC-Semi-Prep-HPLC and the assessment of their potential as fatty acid synthase inhibitors. Int. J. Anal. Chem. 2023, 2023, 11. [Google Scholar] [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Hung, T.W.; Yang, S.F.; Tsai, Y.L.; Yang, J.T.; Lin, C.L.; Hsieh, Y.H. Asiatic acid from Centella asiatica exert anti-invasive ability in human renal cancer cells by modulation of ERK/p38MAPK-mediated MMP15 expression. Phytomedicine 2022, 100, 154036. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chuang, Y.C.; Lo, Y.S.; Lin, C.C.; Hsi, Y.T.; Hsieh, M.J.; Chen, M.K. Asiatic acid, extracted from Centella asiatica and induces apoptosis pathway through the phosphorylation p38 mitogen-activated protein kinase in cisplatin-resistant nasopharyngeal carcinoma cells. Biomolecules 2020, 10, 184. [Google Scholar] [CrossRef]
- Yun, X.; Zhang, Q.; Fang, Y.; Lv, C.; Chen, Q.; Chu, Y.; Zhu, Y.; Wei, Z.; Xia, Y.; Dai, Y. Madecassic acid alleviates colitis-associated colorectal cancer by blocking the recruitment of myeloid-derived suppressor cells via the inhibition of IL-17 expression in γδT17 cells. Biochem. Pharmacol. 2022, 202, 115138. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H.H.W. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends. Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, X. Potential therapeutic targets and biological mechanisms of Centella asiatica on hepatic fibrosis: A study of network pharmacology. Ann. Transl. Med. 2021, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.L.; Zhou, L.P.; Huang, X.Z.; Li, S.; Guan, S.; Li, J.; Zhang, L. Asiatic acid alleviates liver fibrosis via multiple signaling pathways based on integrated network pharmacology and lipidomics. Eur. J. Pharmacol. 2022, 931, 175193. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tang, L.; Shan, Y.; Yu, M.; Sheng, L.; Huang, L.; Cao, H.; Dai, H.; Wang, F.; Zhao, J.; et al. TMT quantitative proteomics and network pharmacology reveal the mechanism by which asiaticoside regulates the JAK2/STAT3 signaling pathway to inhibit peritoneal fibrosis. J. Ethnopharmacol. 2023, 309, 116343. [Google Scholar] [CrossRef]
- Lu, J.; Chen, C.; Gai, R.; Qiu, H.; Wu, Y.; He, Q.; Yang, X. Protective effects and possible mechanisms of Centella asiatica (L.) urban extract against acute and chronic liver injury: Evidence from in vivo and in vitro studies. Phytother. Res. 2021, 35, 2785–2796. [Google Scholar] [CrossRef]
- Ding, B.; Niu, W.; Wang, S.; Zhang, F.; Wang, H.; Chen, X.; Chen, S.; Ma, S.; Kang, W.; Wang, M.; et al. Centella asiatica (L.) Urb. attenuates cardiac hypertrophy and improves heart function through multi-level mechanisms revealed by systems pharmacology. J. Ethnopharmacol. 2022, 291, 115106. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Wu, D.; Zhou, Y.; Duan, B. Centella triterpenes cream as a potential drug for the treatment of hypertrophic scar through inhibiting the phosphorylation of STAT3: A network pharmacology analysis and in vitro experiments. J. Cosmet. Dermatol. 2023, 22, 3511–3519. [Google Scholar] [CrossRef]
- Lusardi, M.; Wehrle-Haller, B.; Sidibe, A.; Ponassi, M.; Iervasi, E.; Rosano, C.; Brullo, C.; Spallarossa, A. Novel 5-aminopyrazoles endowed with anti-angiogenetic properties: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2023, 260, 115727. [Google Scholar] [CrossRef]
- Tian, M.; Chen, K.; Huang, J.; Chu, D.; Li, J.; Huang, K.; Ma, C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother. Res. 2021, 35, 6389–6400. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, H.; Shi, X.; Zhao, J.; Chen, Y.; Wu, F.; Yang, J.; Li, X. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway. Int. Immunopharmacol. 2020, 79, 106109. [Google Scholar] [CrossRef]
- Feng, X.; Huang, D.; Lin, D.; Zhu, L.; Zhang, M.; Chen, Y.; Wu, F. Effects of asiaticoside treatment on the survival of random skin flaps in rats. J. Investig. Surg. 2021, 34, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223–8230. [Google Scholar] [CrossRef]
- Fard, S.E.; Tafvizi, F.; Torbati, M.B. Silver nanoparticles biosynthesised using Centella asiatica leaf extract: Apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. 2018, 12, 994–1002. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, M.Y.; Wang, Z.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Jin, C.; Xu, G.; Piao, L.; Piao, H.; et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine 2020, 68, 153172. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.; Park, Y.; Yang, Y.M. Nobiletin inhibits angiogenesis by regulating Src/FAK/STAT3-mediated signaling through PXN in ER+ breast cancer cells. Int. J. Mol. Sci. 2017, 18, 935. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, X.; Sun, Q.; Jiang, Y.; Zhang, W.; Luo, J.; Li, Y. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. Biomed. Pharmacother. 2020, 125, 109746. [Google Scholar] [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Ranjan, K.; Chhipa, A.S.; Tiwari, B.S.; Pathak, C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol. Cell. Biochem. 2021, 476, 585–598. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Chen, Y.; Li, C.; Wang, X.; Zhang, Y.; Li, S.; Yang, M. Utilizing network pharmacology and experimental validation to explore the potential molecular mechanisms of BanXia-YiYiRen in treating insomnia. Bioengineered 2022, 13, 3148–3170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Xu, X.X.; Tan, B.Z.; Zhang, Z.; Zhou, X.D. MicroRNA-29b inhibits angiogenesis by targeting VEGFA through the MAPK/ERK and PI3K/Akt signaling pathways in endometrial carcinoma. Cell. Physiol. Biochem. 2017, 41, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.J.; Esposito, E.; Hayakawa, K.; Mandaville, E.; Smith, R.A.A.; Guo, S.; Niu, W.; Wong, P.T.; Cool, S.M.; Lo, E.H.; et al. Vascular endothelial growth factor 165-binding heparan sulfate promotes functional recovery from cerebral ischemia. Stroke 2020, 51, 2844–2853. [Google Scholar] [CrossRef] [PubMed]












| Number | Name | Molecular Formula | PubChem ID | Genes Related to Angiogenesis |
|---|---|---|---|---|
| CA1 | Quercetin | C15H10O7 | 5280343 | 86 |
| CA2 | Apigenin | C15H10O5 | 5280443 | 37 |
| CA3 | Ursolic acid | C30H48O3 | 64945 | 37 |
| CA4 | Madecassoside | C48H78O20 | 45356919 | 23 |
| CA5 | Asiaticoside | C48H78O19 | 11954171 | 21 |
| CA6 | Madecassic acid | C30H48O6 | 73412 | 21 |
| CA7 | Asiatic acid | C30H48O5 | 119034 | 21 |
| CA8 | Asiaticoside B | C48H78O20 | 91618002 | 21 |
| CA9 | Madasiatic acid | C30H48O5 | 162998158 | 19 |
| CA10 | Protobassic acid | C30H48O6 | 21576541 | 16 |
| CA11 | 4,5-Dicaffeoylquinic acid | C25H24O12 | 6474309 | 14 |
| CA12 | 3,4-Dicaffeoylquinic acid | C25H24O12 | 5281780 | 11 |
| CA13 | Chlorogensaure | C16H18O9 | 12310830 | 7 |
| CA14 | Stachyose | C24H42O21 | 439531 | 7 |
| CA15 | Neochlorogenic acid | C16H18O9 | 7067333 | 7 |
| CA16 | Heriguard | C16H18O9 | 1794427 | 7 |
| CA17 | 3,5-Dicaffeoylquinic acid | C25H24O12 | 6474310 | 6 |
| CA18 | Troxerutin | C27H30O16 | 5280805 | 5 |
| CA19 | 3,5-Dicaffeoyl-4-maloNylquinic acid | C28H26O15 | 44544976 | 5 |
| CA20 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 6474640 | 5 |
| CA21 | Cynarine | C25H24O12 | 5281769 | 4 |
| CA22 | Castilliferol | C24H16O8 | 10526707 | 2 |
| CA23 | Sitosterol | C29H50O | 12303645 | 1 |
| CA24 | Chlorogenic | C16H18O9 | 94854309 | 1 |
| CA25 | Castillicetin | C24H16O10 | 102394640 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Li, Y.; Wang, B.; Liu, J.; Yang, Y.; Quan, Q.; An, Q.; Liang, R.; Liu, C.; Yang, C. Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation. Molecules 2024, 29, 362. https://doi.org/10.3390/molecules29020362
Zhao B, Li Y, Wang B, Liu J, Yang Y, Quan Q, An Q, Liang R, Liu C, Yang C. Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation. Molecules. 2024; 29(2):362. https://doi.org/10.3390/molecules29020362
Chicago/Turabian StyleZhao, Bingtian, Yuanyuan Li, Binya Wang, Jing Liu, Yang Yang, Qianghua Quan, Quan An, Rong Liang, Chunhuan Liu, and Cheng Yang. 2024. "Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation" Molecules 29, no. 2: 362. https://doi.org/10.3390/molecules29020362
APA StyleZhao, B., Li, Y., Wang, B., Liu, J., Yang, Y., Quan, Q., An, Q., Liang, R., Liu, C., & Yang, C. (2024). Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation. Molecules, 29(2), 362. https://doi.org/10.3390/molecules29020362