Bioactivities and Mechanisms of Action of Sinomenine and Its Derivatives: A Comprehensive Review
Abstract
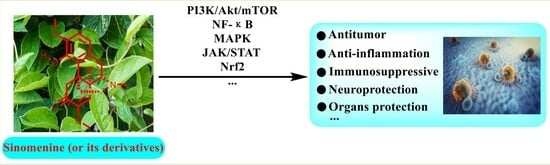
:1. Introduction
2. Methodology
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Antitumor Activity
3.1. Antitumor Activity of SIN When Used Alone
3.2. Antitumor Activity of Sinomenine Hydrochloride
3.3. Combination Strategies for Synergetic Enhancement between SIN and Other Drugs
3.4. Antitumor Activity of SIN Derivatives
4. Anti-Inflammatory Activity and Analgesic Activity
4.1. Anti-Inflammatory Activity and Analgesic Activity of SIN
4.2. Anti-Inflammatory Activity and Analgesic Activity of Compounds Derived from SIN
5. Neuroprotective Activity of SIN
6. Immunosuppression Activity
7. Anti-Depression Activity of SIN
8. Anti-Sepsis Activity of SIN
9. Organs Protection
9.1. Kidney Protection
9.2. Osseous Tissue Protection
9.3. Brain Tissue Protection
9.4. Cardiovascular Tissue Protection
9.5. Liver Protection
9.6. Respiratory Protection
10. Antioxidant Activity
11. Drug–Drug Interaction
12. Other Activities of SIN
13. Other Bioactivities of SIN Derivatives
14. Conclusions or Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ahmed, S.; Alam, W.; Aschner, M.; Alsharif, K.F.; Albrakati, A.; Saso, L.; Khan, H. Natural products targeting the ATR-CHK1 signaling pathway in cancer therapy. Biomed. Pharmacother. 2022, 155, 113797. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Xu, X.-L.; Lan, J.-X.; Huang, H.; Dai, W.; Peng, X.-P.; Liu, S.-L.; Chen, W.-M.; Huang, L.-J.; Liu, J.; Li, X.-J.; et al. Synthesis, biological activity and mechanism of action of novel allosecurinine derivatives as potential antitumor agents. Bioorganic Med. Chem. 2023, 82, 117234. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, L.; Guan, X.; Qin, J.J. Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action. Pharmacol. Res. 2022, 182, 106357. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Huang, H.; Wu, X.-Q.; Lan, J.-X. Bioactivities and mechanism of action of securinega alkaloids derivatives reported prior to 2022. Biomed. Pharmacother. 2023, 158, 114190. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Huang, L.-J.; Huang, H.; Liu, S.-L.; Dai, W.; Li, Z.-M.; Zhang, Z.-Y.; Xin, S.-Y.; Wang, J.-Y.; Zhang, Z.-Y.; et al. Bioactivities and Mechanisms of Action of Diphyllin and Its Derivatives: A Comprehensive Systematic Review. Molecules 2023, 28, 7874. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Han, J.Y.; Iqbal, O.; Liang, A.H. Research Advances and Prospects on Mechanism of Sinomenin on Histamine Release and the Binding to Histamine Receptors. Int. J. Mol. Sci. 2018, 20, 70. [Google Scholar] [CrossRef]
- Yang, S.; Ning, F.; Li, J.; Guo, D.; Zhang, L.; Cui, R.; Liu, Y. Therapeutic Effect Analysis of Sinomenine on Rat Cerebral Ischemia–Reperfusion Injury. J. Stroke Cerebrovasc. Dis. 2016, 25, 1263–1269. [Google Scholar] [CrossRef]
- Qiu, J.; Yan, Z.; Tao, K.; Li, Y.; Li, Y.; Li, J.; Dong, Y.; Feng, D.; Chen, H. Sinomenine activates astrocytic dopamine D2 receptors and alleviates neuroinflammatory injury via the CRYAB/STAT3 pathway after ischemic stroke in mice. J. Neuroinflamm. 2016, 13, 263. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Co-amorphous systems of sinomenine with nonsteroidal anti-inflammatory drugs: A strategy for solubility improvement, sustained release, and drug combination therapy against rheumatoid arthritis. Int. J. Pharm. 2021, 606, 120894. [Google Scholar] [CrossRef]
- Gao, L.N.; Zhong, B.; Wang, Y. Mechanism Underlying Antitumor Effects of Sinomenine. Chin. J. Integr. Med. 2019, 25, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Zhang, S.J.; Liao, J.J.; Gong, Z.P.; Chai, X.; Lyu, H. Towards Better Sinomenine-Type Drugs to Treat Rheumatoid Arthritis: Molecular Mechanisms and Structural Modification. Molecules 2022, 27, 8645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fan, W.; Gao, T.; Li, T.; Yin, Z.; Guo, H.; Wang, L.; Han, Y.; Jiang, J.D. Analgesic Mechanism of Sinomenine against Chronic Pain. Pain Res. Manag. 2020, 2020, 1876862. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Zhou, Y.; Liu, Z.; Zhou, Z.; Huang, J. A review on pharmacokinetics of sinomenine and its anti-inflammatory and immunomodulatory effects. Int. Immunopharmacol. 2023, 119, 110227. [Google Scholar] [CrossRef]
- Hong, H.; Lu, X.; Lu, Q.; Huang, C.; Cui, Z. Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front. Pharmacol. 2022, 13, 1015035. [Google Scholar] [CrossRef]
- Zhang, M.W.; Wang, X.H.; Shi, J.; Yu, J.G. Sinomenine in Cardio-Cerebrovascular Diseases: Potential Therapeutic Effects and Pharmacological Evidences. Front. Cardiovasc. Med. 2021, 8, 749113. [Google Scholar] [CrossRef]
- Li, D.; Zhong, Z.; Ko, C.N.; Tian, T.; Yang, C. From mundane to classic: Sinomenine as a multi-therapeutic agent. Br. J. Pharmacol. 2023; early view. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Li, X.J.; Yue, P.Y.; Ha, W.Y.; Wong, D.Y.; Tin, M.M.; Wang, P.X.; Wong, R.N.; Liu, L. Effect of sinomenine on gene expression of the IL-1 beta-activated human synovial sarcoma. Life Sci. 2006, 79, 665–673. [Google Scholar] [CrossRef]
- Ou, Y.Q.; Chen, L.H.; Li, X.J.; Lin, Z.B.; Li, W.D. Sinomenine influences capacity for invasion and migration in activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, and CD147. Acta Pharmacol. Sin. 2009, 30, 435–441. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, L.; Zhang, W.; Qu, D.; Xu, X.; Yang, Y.; Li, S. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol. Med. Rep. 2010, 3, 51–56. [Google Scholar]
- Fan, J.; Wang, J.C.; Chen, Y.; Fang, H.; Lou, B.; Xie, J.J.; Zhu, L.F.; Tong, X.M. Sinomenine induces apoptosis of prostate cancer cells by blocking activation of NF-kappa B. Afr. J. Biotechno. 2011, 10, 3480–3487. [Google Scholar]
- Zhou, L.; Luan, H.; Liu, Q.; Jiang, T.; Liang, H.; Dong, X.; Shang, H. Activation of PI3K/Akt and ERK signaling pathways antagonized sinomenine-induced lung cancer cell apoptosis. Mol. Med. Rep. 2012, 5, 1256–1260. [Google Scholar]
- Song, L.; Liu, D.; Zhao, Y.; He, J.; Kang, H.; Dai, Z.; Wang, X.; Zhang, S.; Zan, Y. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem. Biophys. Res. Commun. 2015, 464, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, Y.; Hou, W.; Liu, R.; Qi, X.; Xu, X.; Li, J.; Bao, Y.; Zheng, H.; Hua, B. Sinomenine inhibits A549 human lung cancer cell invasion by mediating the STAT3 signaling pathway. Oncol. Lett. 2016, 12, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ren, H.Y.; Lin, H.Q.; Mao, J.P.; Zhu, T.; Wang, S.D.; Ye, Z.M. Sinomenine prevents metastasis of human osteosarcoma cells via S phase arrest and suppression of tumor-related neovascularization and osteolysis through the CXCR4-STAT3 pathway. Int. J. Oncol. 2016, 48, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiao, Y.; Wang, Z.; Li, T.; Liu, Y.; Li, Y.; Zhao, X.; Wang, D. Sinomenine Hydrochloride Inhibits Human Glioblastoma Cell Growth through Reactive Oxygen Species Generation and Autophagy-Lysosome Pathway Activation: An In Vitro and In Vivo Study. Int. J. Mol. Sci. 2017, 18, 1945. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zheng, L.; Liu, X.; Xing, W.; Liu, X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des. Dev. Ther. 2018, 12, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Maimaiti, M.; Jiao, Y.; Meng, X.; Li, H. Sinomenine Induces G1-Phase Cell Cycle Arrest and Apoptosis in Malignant Glioma Cells Via Downregulation of Sirtuin 1 and Induction of p53 Acetylation. Technol. Cancer Res. Treat. 2018, 17, 1533034618770305. [Google Scholar] [CrossRef]
- Song, L.; Liu, D.; Zhao, Y.; He, J.; Kang, H.; Dai, Z.; Wang, X.; Zhang, S.; Zan, Y.; Xue, X. Sinomenine reduces growth and metastasis of breast cancer cells and improves the survival of tumor-bearing mice through suppressing the SHh pathway. Biomed. Pharmacother. 2018, 98, 687–693. [Google Scholar] [CrossRef]
- Gao, G.; Liang, X.; Ma, W. Sinomenine restrains breast cancer cells proliferation, migration and invasion via modulation of miR-29/PDCD-4 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3839–3846. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Li, F.; Li, W.; Wang, H. Sinomenine exerts antitumour effect in gastric cancer cells via enhancement of miR-204 expression. Basic. Clin. Pharmacol. Toxicol. 2019, 125, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, X.; Zhou, L.; Li, J.; Li, M.; Li, W.; Gao, F. Sinomenine Inhibits Non-Small Cell Lung Cancer via Downregulation of Hexokinases II-Mediated Aerobic Glycolysis. Onco Targets Ther. 2020, 13, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, Q.; Li, M.; Su, J.; Wang, P.; Wang, X.; Yin, Y.; Wang, X.; Zhao, M. Sinomenine Suppresses Development of Hepatocellular Carcinoma Cells via Inhibiting MARCH1 and AMPK/STAT3 Signaling Pathway. Front. Mol. Biosci. 2021, 8, 684262. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, H.; Hu, M.; Liu, C.; Zhao, Y.; Zhang, S.; Liu, D. Sinomenine inhibits hypoxia induced breast cancer side population cells metastasis by PI3K/Akt/mTOR pathway. Bioorg. Med. Chem. 2021, 31, 115986. [Google Scholar] [CrossRef]
- Qu, X.; Yu, B.; Zhu, M.; Li, X.; Ma, L.; Liu, C.; Zhang, Y.; Cheng, Z. Sinomenine Inhibits the Growth of Ovarian Cancer Cells Through the Suppression of Mitosis by Down-Regulating the Expression and the Activity of CDK1. Onco Targets Ther. 2021, 14, 823–834. [Google Scholar] [CrossRef]
- Gao, F.; Niu, Y.; Sun, L.; Li, W.; Xia, H.; Zhang, Y.; Geng, S.; Guo, Z.; Lin, H.; Du, G. Integrating network pharmacology and transcriptomic validation to investigate the efficacy and mechanism of Mufangji decoction preventing lung cancer. J. Ethnopharmacol. 2022, 298, 115573. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, L.; Zhao, J.; Jiao, Y.; Zhang, M.; Xu, G.; Jiang, Y. PI3K/AKT1 Signaling Pathway Mediates Sinomenine-Induced Hepatocellular Carcinoma Cells Apoptosis: An in Vitro and in Vivo Study. Biol. Pharm. Bull. 2022, 45, 614–624. [Google Scholar] [CrossRef]
- Duan, D.; Wang, Y.; Pan, D.; Jin, X.; Yan, Y.; Song, P.; Wang, L.; Xiao, J.; Wang, Z.; Wang, X. Rheumatoid arthritis drug sinomenine induces apoptosis of cervical tumor cells by targeting thioredoxin reductase in vitro and in vivo. Bioorg. Chem. 2022, 122, 105711. [Google Scholar] [CrossRef]
- Song, L.; Tang, L.; Lu, D.; Hu, M.; Liu, C.; Zhang, H.; Zhao, Y.; Liu, D.; Zhang, S. Sinomenine Inhibits Vasculogenic Mimicry and Migration of Breast Cancer Side Population Cells via Regulating miR-340-5p/SIAH2 Axis. Biomed. Res. Int. 2022, 2022, 4914005. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Dai, Y.; Xia, Y.F. Sinomenine Ameliorates Colitis-Associated Cancer by Modulating Lipid Metabolism via Enhancing CPT1A Expression. Metabolites 2022, 12, 946. [Google Scholar] [CrossRef]
- Lu, X.L.; Zeng, J.; Chen, Y.L.; He, P.M.; Wen, M.X.; Ren, M.D.; Hu, Y.N.; Lu, G.F.; He, S. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. Int. J. Oncol. 2013, 42, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Ren, Y.; Zhang, L.; Tang, X.J.; Zhang, H.M.; Zhao, C.Q.; Liu, P.J.; Zhang, J.M.; He, J.J. MAPK signaling mediates sinomenine hydrochloride-induced human breast cancer cell death via both reactive oxygen species-dependent and -independent pathways: An in vitro and in vivo study. Cell Death Dis. 2014, 5, e1356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, L.; Mao, J.; Liu, K.; Fan, W.; Liu, J.; Zhang, Z.; Li, Q. Sinomenine hydrochloride attenuates the proliferation, migration, invasiveness, angiogenesis and epithelial-mesenchymal transition of clear-cell renal cell carcinoma cells via targeting Smad in vitro. Biomed. Pharmacother. 2017, 96, 1036–1044. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiao, Y.; Liu, Y.; Zhang, M.; Wang, Z.; Li, Y.; Li, T.; Zhao, X.; Wang, D. Sinomenine Hydrochloride Inhibits the Metastasis of Human Glioblastoma Cells by Suppressing the Expression of Matrix Metalloproteinase-2/-9 and Reversing the Endogenous and Exogenous Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2018, 19, 844. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y.; Yang, N.; Zhang, H.; Zhang, F.; Chen, H.Q.; Meng, J.Q.; Zhang, S.Y.; Li, W. Sinomenine-loaded microcapsules fabricated by phase reversion emulsification-drying in liquid method: An evaluation of process parameters, characterization, and released properties. J. Bioact. Compat. Pol. 2018, 33, 382–396. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, Y.; Zhao, Y.; Zhou, C.; Qian, Y.; Hegde, M.L.; Wang, H.; Han, S. Sinomenine hydrochloride sensitizes cervical cancer cells to ionizing radiation by impairing DNA damage response. Oncol. Rep. 2018, 40, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Jin, L.; Gong, T.; Pan, S.; Zheng, S.; Zhang, X.; Yang, T.; Sun, Y.; Wang, Y.; Guo, J.; et al. Effect of sinomenine hydrochloride on radiosensitivity of esophageal squamous cell carcinoma cells. Oncol. Rep. 2018, 39, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, J.; Li, R.; Ma, Z.; Tao, Y.; Zhang, Q.; Wang, Z. Effects of Tumor-Derived DNA on CXCL12-CXCR4 and CCL21-CCR7 Axes of Hepatocellular Carcinoma Cells and the Regulation of Sinomenine Hydrochloride. Front. Oncol. 2022, 12, 901705. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A.; Jia, X.; Li, X.; Liang, Y.; Liu, Y.; Xie, X.; Qu, X.; Wang, Q.; Zhang, Y.; et al. Sinomenine Hydrochloride Promotes TSHR-Dependent Redifferentiation in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 10709. [Google Scholar] [CrossRef]
- Li, R.Z.; Guan, X.X.; Wang, X.R.; Bao, W.Q.; Lian, L.R.; Choi, S.W.; Zhang, F.Y.; Yan, P.Y.; Leung, E.L.H.; Pan, H.D.; et al. Sinomenine hydrochloride bidirectionally inhibits progression of tumor and autoimmune diseases by regulating AMPK pathway. Phytomedicine 2023, 114, 154751. [Google Scholar] [CrossRef]
- Tong, X.M.; Zhang, J.Y.; Yuedi, S.; Xie, J.J.; Jin, J. Sinomenine enhanced aclarubicin-induced apoptosis by blocking NF-kappa B pathway in HL-60 cells. J. Med. Plants Res. 2011, 5, 635–643. [Google Scholar]
- Liao, F.; Yang, Z.; Lu, X.; Guo, X.; Dong, W. Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in vitro and in vivo. Oncol. Lett. 2013, 6, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, J.; Gui, S.; Chu, X. Preparation, Synergism, and Biocompatibility of in situ Liquid Crystals Loaded with Sinomenine and 5-Fluorouracil for Treatment of Liver Cancer. Int. J. Nanomed. 2021, 16, 3725–3739. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Meng, Y.; Xu, X.F.; Huang, Z.X.; Liu, J.; Wang, Z.Y.; Lin, J.; Chen, W.M. Novel virosecurinine bivalent mimetics as potent reversal agents against P-glycoprotein-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 183, 111726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, Z.J.; Chang, J.Y.; Zhang, Z.F.; Chu, R.; Li, Y.L.; Dai, K.H.; Mo, G.Q.; Chang, Q.Y. Sinomenine sensitizes multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by downregulation of MDR-1 expression. PLoS ONE 2014, 9, e98560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Wang, T. Overcoming cisplatin resistance of human lung cancer by sinomenine through targeting the miR-200a-3p-GLS axis. J. Chemother. 2022, 35, 357–366. [Google Scholar] [CrossRef]
- Vieregge, B.; Resch, K.; Kaever, V. Synergistic effects of the alkaloid sinomenine in combination with the immunosuppressive drugs tacrolimus and mycophenolic acid. Planta Med. 1999, 65, 80–82. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, Y.; Ding, C.Z. A combination of sinomenine and methotrexate reduces joint damage of collagen induced arthritis in rats by modulating osteoclast-related cytokines. Int. Immunopharm. 2014, 18, 135–141. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Nishimura, K.; Ikuta, H.; Takeya, K.; Itokawa, H. Syntheses of Antitumor Morphinane Alkaloids, Sinococuline and 6-Epi-Sinococuline, 7-Epi-Sinococuline, and 6-Epi-7-Epi-Sinococuline, from Sinomenine. J. Org. Chem. 1995, 60, 4549–4558. [Google Scholar] [CrossRef]
- Deng, Z.S.; Li, J.X.; Teng, P.; Li, P.; Sun, X.R. Biocatalyzed cross-coupling of sinomenine and guaiacol by Antrodiella semisupina. Org. Lett. 2008, 10, 1119–1122. [Google Scholar] [CrossRef]
- Deng, Z.S.; Zhao, D.; Hu, Y.; Li, J.X.; Zou, K.; Wang, J.Z. Biocatalyzed cross-coupling of sinomenine and 1,2-dihydroxybenzene by Coriolus unicolor. Chin. Chem. Lett. 2012, 23, 321–324. [Google Scholar] [CrossRef]
- Wei, C.J.; Xu, F.; Shi, M.J.; Hu, J.W.; Wang, J.J.; Zhen, B.; Wang, X.; Ji, T.F.; Wang, J.H.; Du, G.H. Synthesis and antitumor activities of sinomenine derivatives on rings A and C. J. Asian Nat. Prod. Res. 2018, 20, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Cai, H.; Lei, H.; Ma, C.M.; Yang, L.; Xu, H.; Zhu, Q.; Yao, Z.; Wu, Y. YL064 directly inhibits STAT3 activity to induce apoptosis of multiple myeloma cells. Cell Death Discov. 2018, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Cai, H.; Lei, H.; Ma, C.M.; Yang, L.; Xu, H.; Zhu, Q.; Yao, Z.; Wu, Y. Sinomenine derivative YL064: A novel STAT3 inhibitor with promising anti-myeloma activity. Cell Death Dis. 2018, 9, 1093. [Google Scholar] [CrossRef]
- Li, S.; Gao, M.; Nian, X.; Zhang, L.; Li, J.; Cui, D.; Zhang, C.; Zhao, C. Design, Synthesis, Biological Evaluation and Silico Prediction of Novel Sinomenine Derivatives. Molecules 2021, 26, 3466. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Xu, H.; Liu, J.; Ren, L.; Yang, Y.; Li, S.; Wang, J.; Ji, T.; Du, G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 2021, 11, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, H.; Chen, F.; Deng, J.; Yin, L.; He, B.; Hu, Q.; Wang, T. Sinomenine Derivatives: Synthesis, Antitumor Activity, and Apoptotic Induction in MCF-7 Cells via IL-6/PI3K/Akt/NF-kappaB Signaling Pathway. ChemMedChem 2022, 17, e202200234. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, B.; Hou, Y.; Liu, L.; Sun, J.; Xu, F.; Li, D.; Hua, H. Anti-breast cancer sinomenine derivatives via mechanisms of apoptosis induction and metastasis reduction. J. Enzym. Inhib. Med. Chem. 2022, 37, 1870–1883. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Liu, L.; Buchner, E.; Beitze, D.; Schmidt-Weber, C.B.; Kaever, V.; Emmrich, F.; Kinne, R.W. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int. J. Immunopharmacol. 1996, 18, 529–543. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Guo, Z.; Liu, Q.; Chen, T.; Wang, X.; Cao, X. Requirement for ERK activation in sinomenine-induced apoptosis of macrophages. Immunol. Lett. 2005, 98, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kok, T.W.; Yue, P.Y.; Mak, N.K.; Fan, T.P.; Liu, L.; Wong, R.N. The anti-angiogenic effect of sinomenine. Angiogenesis 2005, 8, 3–12. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Huang, W.; Zhou, X.; Wang, M.; Zhong, B.; Peng, D. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J. Ethnopharmacol. 2005, 98, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.H.; Qin, G.W.; Wang, R.; Tang, X.C. Morphinane alkaloids with cell protective effects from Sinomenium acutum. J. Nat. Prod. 2005, 68, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.L.; Cai, Y.X.; Xu, S.S.; Wu, S.F.; Li, Y.L.; Chen, X.L.; Kong, L.Y.; Luo, J.G. New N-oxide alkaloids from the stems of Sinomenium acutum. Fitoterapia 2023, 165, 105404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xia, B.; Guo, Q.; Zhang, L.; Wang, F.; Jiang, L.; Wang, Z.; Zhang, Y.; Li, C. Sinomenine attenuates 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in mice. Int. Immunopharmacol. 2007, 7, 604–611. [Google Scholar] [CrossRef]
- Wang, A.L.; Li, Z.; Yuan, M.; Yu, A.C.; Zhu, X.; Tso, M.O. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products. Int. Immunopharmacol. 2007, 7, 1552–1558. [Google Scholar] [CrossRef]
- Wang, M.H.; Chang, C.K.; Cheng, J.H.; Wu, H.T.; Li, Y.X.; Cheng, J.T. Activation of opioid mu-receptor by sinomenine in cell and mice. Neurosci. Lett. 2008, 443, 209–212. [Google Scholar] [CrossRef]
- Zhou, H.; Wong, Y.F.; Wang, J.; Cai, X.; Liu, L. Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in rats. Biochem. Biophys. Res. Commun. 2008, 376, 352–357. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Hou, W.; Wang, D.; Li, F.; Zhang, Y.; Yuan, F. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int. Immunopharmacol. 2009, 9, 894–899. [Google Scholar] [CrossRef]
- Huang, B.D.; He, A.S.; Fu, M.; Sheng, P.Y.; Liao, W.M. Sinomenine suppresses expression of interleukin-1beta-induced matrix metalloproteinases in human osteoarthritic chondrocytes. J. Med. Plants Res. 2010, 4, 1830–1836. [Google Scholar]
- Ju, X.D.; Deng, M.; Ao, Y.F.; Yu, C.L.; Wang, J.Q.; Yu, J.K.; Cui, G.Q.; Hu, Y.L. Protective Effect of Sinomenine on Cartilage Degradation and Chondrocytes Apoptosis. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2010, 130, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Li, W.; Li, X.; Lin, Z.; Li, M. Sinomenine reduces invasion and migration ability in fibroblast-like synoviocytes cells co-cultured with activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, CD147. Rheumatol. Int. 2011, 31, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Wong, C.K.; Leung, P.C.; Fung, K.P.; Lau, C.B.; Lau, C.P.; Li, E.K.; Tam, L.S.; Lam, C.W. Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-α-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J. Ethnopharmacol. 2011, 137, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Kang, O.H.; Kim, S.B.; Mun, S.H.; Park, C.B.; Kim, Y.G.; Kim, Y.I.; Lee, Y.S.; Han, S.H.; Keum, J.H.; et al. Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 Cells. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1184–1191. [Google Scholar]
- Li, X.; He, L.; Hu, Y.; Duan, H.; Li, X.; Tan, S.; Zou, M.; Gu, C.; Zeng, X.; Yu, L.; et al. Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS ONE 2013, 8, e74274. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhu, S.; Zhou, R.; Yi, F.; Bing, Y.; Huang, S.; Wang, Z.; Wang, C.; Xia, B. Effects of sinomenine on the expression of microRNA-155 in 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. PLoS ONE 2013, 8, e73757. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Yao, R.B.; Zhao, L.J.; Shen, S.Y.; Zhao, Z.M.; Cai, H. Sinomenine decreases MyD88 expression and improves inflammation-induced joint damage progression and symptoms in rat adjuvant-induced arthritis. Inflammation 2013, 36, 1136–1144. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Zhu, J.; Fang, T.; Zhang, W.; Li, J.X. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci. Rep. 2014, 4, 7270. [Google Scholar] [CrossRef]
- Yi, L.; Luo, J.F.; Xie, B.B.; Liu, J.X.; Wang, J.Y.; Liu, L.; Wang, P.X.; Zhou, H.; Dong, Y. α7 Nicotinic Acetylcholine Receptor is a Novel Mediator of Sinomenine Anti-Inflammation Effect in Macrophages Stimulated by Lipopolysaccharide. Shock 2015, 44, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Liu, M.X.; Wang, E.P.; Lin, Z.; Lv, G.F.; Chen, X. Effect of sinomenine on the expression of rheumatoid arthritis fibroblast-like synoviocytes MyD88 and TRAF6. Genet. Mol. Res. 2015, 14, 18928–18935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Y.; Mao, L.; Liu, C.; Jiang, B.; Zhang, W.; Li, J.X. Antinociceptive effects of sinomenine in a rat model of postoperative pain. Br. J. Pharmacol. 2016, 173, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.R.; Liu, X.J.; Li, Y.L.; Men, X.; Zeng, X.L. Sinomenine attenuates airway inflammation and remodeling in a mouse model of asthma. Mol. Med. Rep. 2016, 13, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Yuan, X.; Dou, Y.; Wu, X.; Wang, Y.; Xia, Y.; Dai, Y. Sinomenine induces the generation of intestinal Treg cells and attenuates arthritis via activation of aryl hydrocarbon receptor. Lab. Investig. 2016, 96, 1076–1086. [Google Scholar] [CrossRef]
- Rao, S.; Liu, S.; Zou, L.; Jia, T.; Zhao, S.; Wu, B.; Yi, Z.; Wang, S.; Xue, Y.; Gao, Y.; et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X(3) receptor in dorsal root ganglia. Purinergic Signal. 2017, 13, 227–235. [Google Scholar] [CrossRef]
- Xiong, H.; Tian, L.; Zhao, Z.; Chen, S.; Zhao, Q.; Hong, J.; Xie, Y.; Zhou, N.; Fu, Y. The sinomenine enteric-coated microspheres suppressed the TLR/NF-kappaB signaling in DSS-induced experimental colitis. Int. Immunopharmacol. 2017, 50, 251–262. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Wen, L.; He, K. Study on the anti-endotoxin effect of sinomenine using an Agilent genome array. Qjm-An Int. J. Med. 2018, 111, 171–178. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Y.; Shang, W.; Zhao, Z.M.; Qian, X.; Zhang, B.B.; Cai, H. Sinomenine relieves oxygen and glucose deprivation-induced microglial activation via inhibition of the SP1/miRNA-183-5p/IκB-α signaling pathway. Cell. Mol. Biol. 2018, 64, 140–147. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhu, W.; Ma, C.; Ruan, J.; Long, H.; Wang, Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front. Immunol. 2018, 9, 2228. [Google Scholar] [CrossRef]
- Yue, M.; Zhang, X.; Dou, Y.; Wei, Z.; Tao, Y.; Xia, Y.; Dai, Y. Gut-Sourced Vasoactive Intestinal Polypeptide Induced by the Activation of α7 Nicotinic Acetylcholine Receptor Substantially Contributes to the Anti-inflammatory Effect of Sinomenine in Collagen-Induced Arthritis. Front. Pharmacol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; He, X.; Fan, S. Protective Effects of Sinomenine on CFA-Induced Inflammatory Pain in Rats. Med. Sci. Monit. 2018, 24, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Han, J.M.; Han, Y.K.; Chung, H. Anti-inflammatory Effects of Sinomenium Acutum Extract On Endotoxin-induced Uveitis in Lewis Rats. Int. J. Med. Sci. 2018, 15, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, S.; Wan, R.; Chen, Y. Combined treatment with sinomenine and acupuncture on collagen-induced arthritis through the NF-kappaB and MAPK signaling pathway. Oncol. Lett. 2018, 15, 8770–8776. [Google Scholar] [PubMed]
- Zhou, Y.; Liu, H.; Song, J.; Cao, L.; Tang, L.; Qi, C. Sinomenine alleviates dextran sulfate sodium-induced colitis via the Nrf2/NQO-1 signaling pathway. Mol. Med. Rep. 2018, 18, 3691–3698. [Google Scholar] [CrossRef]
- Shen, J.; Yao, R.; Jing, M.; Zhou, Z. Sinomenine Regulates Inflammatory Response and Oxidative Stress via Nuclear Factor kappa B (NF-kappaB) and NF-E2-Related Factor 2 (Nrf2) Signaling Pathways in Ankle Fractures in Children. Med. Sci. Monit. 2018, 24, 6649–6655. [Google Scholar] [CrossRef]
- Chen, S.P.; Sun, J.; Zhou, Y.Q.; Cao, F.; Braun, C.; Luo, F.; Ye, D.W.; Tian, Y.K. Sinomenine attenuates cancer-induced bone pain via suppressing microglial JAK2/STAT3 and neuronal CAMKII/CREB cascades in rat models. Mol. Pain 2018, 14, 1744806918793232. [Google Scholar] [CrossRef]
- Chu, X.Q.; Gui, S.Y.; Lin, X.Y.; Wang, S.M.; Jiang, X.J.; Zhang, Y.Z.; Zhai, H.Y.; Jiang, J.Q. Evaluation of Effects of a Chinese Herb Formula on Adjuvant Induced Arthritis in Rats. Int. J. Pharmacol. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Yan, Z.; Wang, Z.; Fu, X.; Yu, K. Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-kappaB signaling pathways. Int. Immunopharmacol. 2019, 75, 105715. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, C.; Zhang, H. Lipopolysaccharides-mediated injury to chondrogenic ATDC5 cells can be relieved by Sinomenine via downregulating microRNA-192. Phytother. Res. 2019, 33, 1827–1836. [Google Scholar] [CrossRef]
- Song, H.; Wen, J.; Li, H.; Meng, Y.; Zhang, Y.; Zhang, N.; Zheng, W. Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. Int. J. Nanomed. 2019, 14, 3177–3188. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Ma, Q.; Li, Y. Sinomenine retards LPS-elicited inflammation via down-regulating CCAT1 in HaCaT cells. Life Sci. 2019, 233, 116703. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shi, Q.P.; Liu, J.Y.; Lv, Y.J.; Li, J.; Yi, L.; Bai, S.S.; Liu, L.; Wang, P.X.; Zhou, H.; et al. A7 nAChR Expression Is Correlated with Arthritis Development and Inhibited by Sinomenine in Adjuvant-Induced Arthritic Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 3759304. [Google Scholar] [CrossRef]
- Zhu, R.L.; Zhi, Y.K.; Yi, L.; Luo, J.F.; Li, J.; Bai, S.S.; Liu, L.; Wang, P.X.; Zhou, H.; Dong, Y. Sinomenine regulates CD14/TLR4, JAK2/STAT3 pathway and calcium signal via α7nAChR to inhibit inflammation in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2019, 41, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, C.J.; Liu, R.; Barr, G.A. Effects of plant-derived analgesic compounds sinomenine and salvinorin A in infant rats. J. Integr. Med. 2020, 18, 174–180. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Tong, Q.Y. Anti-inflammation Effects of Sinomenine on Macrophages through Suppressing Activated TLR4/NF-kappaB Signaling Pathway. Curr. Med. Sci. 2020, 40, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Xiong, Y.; Tao, W.; Chen, J.; Wang, Z. Sinomenine alleviates lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. Immunopharmacol. Immunotoxicol. 2020, 42, 147–155. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, X.; Qi, J.; Shu, G.; Du, Y.; Ying, X. Sinomenine hydrochloride loaded thermosensitive liposomes combined with microwave hyperthermia for the treatment of rheumatoid arthritis. Int. J. Pharm. 2020, 576, 119001. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Gu, Y.F.; Wang, Z.; Fan, W.M. Sinomenine Inhibited Interleukin-1 beta-Induced Matrix Metalloproteinases Levels via SOCS3 Up-Regulation in SW1353 Cells. Biol. Pharm. Bull. 2020, 43, 1643–1652. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.B.; Wang, Y.Y.; Kou, F.; Lyu, C.M.; Wei, H. Metabolic mechanism and anti-inflammation effects of sinomenine and its major metabolites N-demethylsinomenine and sinomenine-N-oxide. Life Sci. 2020, 261, 118433. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, H.; Jin, J.; Ma, Y.; Leng, Y. Sinomenine alleviates dorsal root ganglia inflammation to inhibit neuropathic pain via the p38 MAPK/CREB signalling pathway. Eur. J. Pharmacol. 2021, 897, 173945. [Google Scholar] [CrossRef]
- Xu, W.; Chen, S.; Wang, X.; Wu, H.; Tahara, K.; Tanaka, S.; Sugiyama, K.; Yamada, H.; Sawada, T.; Hirano, T. Effects of sinomenine on the proliferation, cytokine production, and regulatory T-cell frequency in peripheral blood mononuclear cells of rheumatoid arthritis patients. Drug Dev. Res. 2021, 82, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, M.; Ye, W.; Shan, J.; Zhao, X.; Duan, Y.; Liu, Y.; Unger, B.H.; Cheng, Y.; Zhang, W.; et al. Sinomenine Hydrochloride Ameliorates Fish Foodborne Enteritis via α7nAchR-Mediated Anti-Inflammatory Effect Whilst Altering Microbiota Composition. Front. Immunol. 2021, 12, 766845. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Qu, Y.J.; Li, D.Y.; Yue, S.W. RIP3 Inhibition ameliorates chronic constriction injury-induced neuropathic pain by suppressing JNK signaling. Aging 2021, 13, 24417–24431. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Liu, J.X.; Xie, Y.; Luo, P.; Liu, Z.Q.; Liu, L.; Zhou, H. Suppression of macrophage migration by down-regulating Src/FAK/P130Cas activation contributed to the anti-inflammatory activity of sinomenine. Pharmacol. Res. 2021, 167, 105513. [Google Scholar] [CrossRef]
- Liao, K.; Su, X.; Lei, K.; Liu, Z.; Lu, L.; Wu, Q.; Pan, H.; Huang, Q.; Zhao, Y.; Wang, M.; et al. Sinomenine protects bone from destruction to ameliorate arthritis via activating p62(Thr269/Ser272)-Keap1-Nrf2 feedback loop. Biomed. Pharmacother. 2021, 135, 111195. [Google Scholar] [CrossRef]
- Yi, L.; Ke, J.; Liu, J.; Lai, H.; Lv, Y.; Peng, C.; Zhi, Y.; Du, Q.; Liu, L.; Wang, P.; et al. Sinomenine increases adenosine A(2A) receptor and inhibits NF-kappaB to inhibit arthritis in adjuvant-induced-arthritis rats and fibroblast-like synoviocytes through α7nAChR. J. Leukoc. Biol. 2021, 110, 1113–1120. [Google Scholar] [CrossRef]
- Mariani, F.M.; Martelli, I.; Pistone, F.; Chericoni, E.; Puxeddu, I.; Alunno, A. Pathogenesis of rheumatoid arthritis: One year in review 2023. Clin. Exp. Rheumatol. 2023, 41, 1725–1734. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Gu, W.; Sun, X.; Wang, L.; Tang, L. Sinomenine hydrochloride ameliorates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota composition whilst suppressing the activation of the NLRP3 inflammasome. Exp. Ther. Med. 2021, 22, 1287. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhao, X. Sinomenine attenuates lipopolysaccharide-induced inflammation and apoptosis of WI-38 cells by reducing glutathione S-transferase M1 expression. Chem. Biol. Drug Des. 2022, 102, 434–443. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Zeng, S.L.; Huang, T.Q.; Lin, Y.; Wang, F.F.; Gao, X.J.; Li, J.; Li, P.; Liu, E.H. Sinomenine ameliorates rheumatoid arthritis by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Sci. Bull. 2023, 68, 1540–1555. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Liu, Y.W.; Tan, Y.; Yin, J.; Chen, Y.; Chen, D.; Ni, B. Sinomenine alleviates lipopolysaccharide-induced acute lung injury via a PPARbeta/delta-dependent mechanism. Eur. J. Pharmacol. 2023, 953, 175838. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, Q.; Xu, J.; Huo, G.; Cai, Y.; Geng, S.; Xu, H.; Zhang, J.; Li, H.; Yuan, K.; et al. Sinomenine ameliorates adjuvant-induced arthritis by inhibiting the autophagy/NETosis/inflammation axis. Sci. Rep. 2023, 13, 3933. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Luo, J.; Zhu, Q.; Li, Y.; Yin, S. Synthesis and anti-inflammatory activities investigation of sinomenine derivatives on ring C. Nat. Prod. Res. 2006, 20, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-T.; Zhou, H.-B.; Zou, J.; Yan, L.-C.; Bi, E.-G.; Sun, B.; Yao, Z.-J. Modification of poorly bioactive sinomenine into more potent immunosuppressive agents by embedding of drug-like fragments. Tetrahedron Lett. 2010, 51, 485–488. [Google Scholar] [CrossRef]
- Teng, P.; Liu, H.L.; Deng, Z.S.; Shi, Z.B.; He, Y.M.; Feng, L.L.; Xu, Q.; Li, J.X. Synthesis and biological evaluation of unique stereodimers of sinomenine analogues as potential inhibitors of NO production. Bioorg. Med. Chem. 2011, 19, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, L.Y.; Lou, Y.T.; Bian, C.; Zhou, T.T.; Zhou, H.B.; Liao, H.Z.; Ma, Z.; Yin, D.S.; Chen, A.Z.; et al. Sinomenine derivatives with embedment of nitrogen-containing heterocycles exhibiting potent TNF-α inhibitory activity. Sci. China-Chem. 2012, 55, 2537–2547. [Google Scholar] [CrossRef]
- Chai, X.; Guan, Z.; Yu, S.; Zhao, Q.; Hu, H.; Zou, Y.; Tao, X.; Wu, Q. Design, synthesis and molecular docking studies of sinomenine derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5849–5852. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.T.; Ma, L.Y.; Wang, M.; Yin, D.S.; Zhou, T.T.; Chen, A.Z.; Ma, Z.; Bian, C.; Wang, S.Z.; Yang, Z.Y.; et al. Regio- and stereoselective C-10 beta-H functionalization of sinomenine: An access to more potent immunomodulating derivatives. Tetrahedron 2012, 68, 2172–2178. [Google Scholar] [CrossRef]
- Teng, P.; Liu, H.L.; Zhang, L.; Feng, L.L.; Huai, Y.; Deng, Z.S.; Sun, Y.; Xu, Q.; Li, J.X. Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory agents. Eur. J. Med. Chem. 2012, 50, 63–74. [Google Scholar] [CrossRef]
- Jin, J.; Teng, P.; Liu, H.L.; Wu, J.; Liu, Y.M.; Xu, Q.; Li, J.X. Microfluidics assisted synthesis and bioevaluation of sinomenine derivatives as antiinflammatory agents. Eur. J. Med. Chem. 2013, 62, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Hou, J.; Wang, M.; Ma, L.Y.; Wu, L.L.; Wang, S.Z.; Sun, B.; Yao, Z.J. Regio-controlled synthesis of unsymmetrical pyrazine-fused sinomenine derivatives and discriminate substitution effects on TNF-α inhibitory activity. Tetrahedron 2014, 70, 5475–5482. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Zhao, Y.; Bao, B.H.; Li, J.X. SND-117, a sinomenine bivalent alleviates type II collagen-induced arthritis in mice. Int. Immunopharmacol. 2015, 26, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, J.; Wang, J.; Dong, W.; Peng, Z.; An, D. Anti-inflammatory effects of novel sinomenine derivatives. Int. Immunopharmacol. 2015, 29, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Zhao, C.; Xiao, J.; Wang, J.C. Transdermal Permeation and Anti-Inflammation Activities of Novel Sinomenine Derivatives. Molecules 2016, 21, 1520. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Su, M.; Ling, Y.; Wei, Q.; Pan, F.; Li, J.; Li, J.X.; Zhu, Q. Anti-allodynic effects of N-demethylsinomenine, an active metabolite of sinomenine, in a mouse model of postoperative pain. Eur. J. Pharmacol. 2018, 823, 105–109. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, N.; Ou, Y.; Wei, Q.; Tang, W.; Zheng, M.; Xing, Y.; Li, J.J.; Ling, Y.; Li, J.; et al. N-Demethylsinomenine, an active metabolite of sinomenine, attenuates chronic neuropathic and inflammatory pain in mice. Sci. Rep. 2021, 11, 9300. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Dai, Z.; Zhang, T.; Gu, Y.; Cai, D.; Lu, M.; Zhang, Z.; Zeng, Q.; Shang, B.; Xu, B.; et al. Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory and analgesic agent. RSC Adv. 2022, 12, 30001–30007. [Google Scholar] [CrossRef]
- Finke, A.O.; Pavlova, A.V.; Morozova, E.A.; Tolstikova, T.G.; Shults, E.E. Synthesis of 1,2,3-Triazolyl-Substituted Derivatives of the Alkaloids Sinomenine and Tetrahydrothebaine on Ring A and Their Analgesic Activity. Chem. Nat. Compd. 2022, 58, 895–902. [Google Scholar] [CrossRef]
- Qian, L.; Xu, Z.; Zhang, W.; Wilson, B.; Hong, J.S.; Flood, P.M. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J. Neuroinflamm. 2007, 4, 23. [Google Scholar] [CrossRef]
- Shukla, S.M.; Sharma, S.K. Sinomenine inhibits microglial activation by Abeta and confers neuroprotection. J. Neuroinflamm. 2011, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Li, L.; Li, X.; Wang, Q.; Ding, H.; Wang, X.; Ye, Z.; Wu, L.; Zhang, X.; et al. Sinomenine Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Pathway. Front. Neurosci. 2016, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, M.; Zhang, J.; Cai, Q.; Lu, D.; Li, Y.; Dong, Y.; Zhao, T.; Chen, H. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int. Immunopharmacol. 2016, 40, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; Zhang, Y. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J. Neuroimmunol. 2016, 299, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Ha, T.W.; Hong, J.T.; Oh, K.W. Sinomenine, an Alkaloid Derived from Sinomenium acutum Potentiates Pentobarbital-Induced Sleep Behaviors and Non-Rapid Eye Movement (NREM) Sleep in Rodents. Biomol. Ther. 2017, 25, 586–592. [Google Scholar] [CrossRef]
- Ou, J.; Zhou, Y.; Li, C.; Chen, Z.; Li, H.; Fang, M.; Zhu, C.; Huo, C.; Yung, K.K.; Li, J.; et al. Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes. Molecules 2018, 23, 2370. [Google Scholar] [CrossRef]
- Gao, B.; Wu, Y.; Yang, Y.J.; Li, W.Z.; Dong, K.; Zhou, J.; Yin, Y.Y.; Huang, D.K.; Wu, W.N. Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: Involvement of inhibition of NLRP1 inflammasome. J. Neuroinflamm. 2018, 15, 152. [Google Scholar] [CrossRef]
- Komatsu, T.; Katsuyama, S.; Takano, F.; Okamura, T.; Sakurada, C.; Tsuzuki, M.; Ogawa, K.; Kubota, A.; Morinaga, O.; Tabata, K.; et al. Possible involvement of the mu opioid receptor in the antinociception induced by sinomenine on formalin-induced nociceptive behavior in mice. Neurosci. Lett. 2019, 699, 103–108. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zheng, B.; Tian, N. Sinomenine Attenuates Traumatic Spinal Cord Injury by Suppressing Oxidative Stress and Inflammation via Nrf2 Pathway. Neurochem. Res. 2019, 44, 763–775. [Google Scholar] [CrossRef]
- Singh, D.; Agrawal, A.; Singal, C.M.S.; Pandey, H.S.; Seth, P.; Sharma, S.K. Sinomenine inhibits amyloid beta-induced astrocyte activation and protects neurons against indirect toxicity. Mol. Brain 2020, 13, 30. [Google Scholar] [CrossRef]
- Lin, Y.B.; Li, H.C.; Peng, J.; Li, C.; Zhu, C.; Zhou, Y.T.; Chen, Z.J.; Li, J.; Luo, C.H.; Mo, Z.X. Decrease of morphine-CPP by sinomenine via mediation of tyrosine hydroxylase, NMDA receptor subunit 2B and opioid receptor in the zebrafish brain. Pak. J. Pharm. Sci. 2021, 34, 1659–1665. [Google Scholar] [PubMed]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Fakour, M.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; Khodashenas, V.; Tashakori-Miyanroudi, M.; Roghani, M. Sinomenine Alleviates Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Inhibiting NLRP3 Inflammasome. J. Mol. Neurosci. 2021, 71, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, Y.; Liu, W.; Xie, K. Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke. Exp. Ther. Med. 2021, 21, 647. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Taleahmad, F.; Haddadzadeh-Niri, N.; Joneidi, E.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Sinomenine Attenuates Trimethyltin-Induced Cognitive Decline via Targeting Hippocampal Oxidative Stress and Neuroinflammation. J. Mol. Neurosci. 2022, 72, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Shen, Y.; Sun, M.; Zhang, B.; Dai, J.; Chen, D.; Liu, Z. Effect of regulating macrophage polarization phenotype on intervertebral disc degeneration. Immun. Inflamm. Dis. 2022, 10, e714. [Google Scholar] [CrossRef]
- Bao, X.; He, Y.; Huang, L.; Li, H.; Li, Q.; Huang, Y. Sinomenine exerts a neuroprotective effect on PD mouse model through inhibiting PI3K/AKT/mTOR pathway to enhance autophagy. Int. J. Neurosci. 2022, 1–9, Online ahead of print. [Google Scholar] [CrossRef]
- Chen, J.; Guo, P.; Liu, X.; Liao, H.; Chen, K.; Wang, Y.; Qin, J.; Yang, F. Sinomenine alleviates diabetic peripheral neuropathic pain through inhibition of the inositol-requiring enzyme 1 α–X-box binding protein 1 pathway by downregulating prostaglandin-endoperoxide synthase 2. J. Diabetes Investig. 2023, 14, 364–375. [Google Scholar] [CrossRef]
- Fu, C.; Xin, H.; Qian, Z.; Li, X.; Gao, J.; Fan, Y.; Tang, Y.; Shi, Y.; Li, D.; Wu, H. Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage. Brain Sci. 2023, 13, 716. [Google Scholar] [CrossRef]
- Hojo, H.; Kondo, Y.; Umeda, H.; Tahira, T.; Hashimoto, Y. Effect of sinomenine on antibody responses in mice. J. Immunopharmacol. 1985, 7, 33–42. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhang, J.; Zhao, T.; Zou, L.; Tang, Y.; Zhang, X.; Wu, Y. Sinomenine inhibits B7-H1 and B7-DC expression on human renal tubular epithelial cells. Int. Immunopharmacol. 2005, 5, 1446–1457. [Google Scholar] [CrossRef]
- Shu, L.; Yin, W.; Zhang, J.; Tang, B.; Kang, Y.X.; Ding, F.; Hua, Z.C. Sinomenine inhibits primary CD4+ T-cell proliferation via apoptosis. Cell Biol. Int. 2007, 31, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.Y.; Gu, B.J.; Ji, X.H.; Ding, X.S.; Song, C.J.; Wu, F.C. Sinomenine, an antirheumatic alkaloid, ameliorates clinical signs of disease in the lewis rat model of acute experimental autoimmune encephalolmyelitis. Biol. Pharm. Bull. 2007, 30, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Jin, N.; Xie, Z.; Fei, L.; Jia, Z.; Wu, Y. Sinomenine promotes differentiation but impedes maturation and co-stimulatory molecule expression of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2007, 7, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, Z.; Luo, M.; Lu, C.; Kim, M.H.; Yu, B.; Gu, J. Sinomenine suppresses TNF-α-induced VCAM-1 expression in human umbilical vein endothelial cells. J. Ethnopharmacol. 2007, 114, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Yu, K.; Liu, Y.; Chen, X. Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int. Immunopharmacol. 2007, 7, 637–645. [Google Scholar] [CrossRef]
- Huang, F.; Yamaki, K.; Tong, X.; Fu, L.; Zhang, R.; Cai, Y.; Yanagisawa, R.; Inoue, K.; Takano, H.; Yoshino, S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int. Immunopharmacol. 2008, 8, 502–507. [Google Scholar] [CrossRef]
- Kato, A.; Yasui, M.; Yano, N.; Kawata, Y.; Moriki, K.; Adachi, I.; Hollinshead, J.; Nash, R.J. Alkaloids inhibiting L-histidine decarboxylase from Sinomenium acutum. Phytochem. Lett. 2009, 2, 77–80. [Google Scholar] [CrossRef]
- Huang, L.F.; Li, T.; Zhou, H.; Qiu, P.; Wu, J.L.; Liu, L. Sinomenine potentiates degranulation of RBL-2H3 basophils via up-regulation of phospholipase A(2) phosphorylation by Annexin A1 cleavage and ERK phosphorylation without influencing on calcium mobilization. Int. Immunopharmacol. 2015, 28, 945–951. [Google Scholar] [CrossRef]
- Wang, N.; Liu, R.; Liu, Y.; Zhang, R.; He, L. Sinomenine potentiates P815 cell degranulation via upregulation of Ca2+ mobilization through the Lyn/PLCgamma/IP3R pathway. Int. J. Immunopathol. Pharmacol. 2016, 29, 676–683. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ma, L. Depression and cardiovascular disease in elderly: Current understanding. J. Clin. Neurosci. 2018, 47, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Jiang, B.; Chen, K.; Li, W.; Wang, H. The antidepressant-like effects of sinomenine in mice: A behavioral and neurobiological characterization. Behav. Pharmacol. 2018, 29, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, S.; Wang, Z.; Guo, Y.; Pan, W.; Shen, Z. Anti-Depressant-Like Effect of Sinomenine on Chronic Unpredictable Mild Stress-Induced Depression in a Mouse Model. Med. Sci. Monit. 2018, 24, 7646–7653. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gao, M.; Wang, W.; Lang, Y.; Tong, Z.; Wang, K.; Zhang, H.; Chen, G.; Liu, M.; Yao, Y.; et al. Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy. Int. J. Mol. Sci. 2015, 16, 2559–2573. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Chen, Q.; Guo, M.; Liu, S.; Liu, J.; Wang, J.; Huang, F. Sinomenine attenuates septic-associated lung injury through the Nrf2-Keap1 and autophagy. J. Pharm. Pharmacol. 2020, 72, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yang, X.; Wang, W.; Wang, Z.; Wu, J.; Huang, F. Sinomenine ameliorates septic acute lung injury in mice by modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2 pathway. Eur. J. Pharmacol. 2021, 912, 174581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, R.; Xia, Z.K.; Ren, X.G.; Zhang, L.W.; Liang, Y.H.; Liu, G.L. Protective effects of sinomenine against doxorubicin-induced nephrosis in rats. J. Asian Nat. Prod. Res. 2012, 14, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Guan, R.; Song, S.; Zhang, M.; Liu, F.; Guo, M.; Guo, W.; Yu, Q.; Zhang, L.; Wang, Q. Sinomenine protects mice against ischemia reperfusion induced renal injury by attenuating inflammatory response and tubular cell apoptosis. Int. J. Clin. Exp. Pathol. 2013, 6, 1702–1712. [Google Scholar]
- Lyu, X.H.; Yang, Y.N.; Wan, Z.H.; Ma, Y.Q.; Leng, Y.F. Sinomenine protects the kidney from ischemia reperfusion-induced apoptosis via up-regulation of microRNA-124 expression. Int. J. Clin. Exp. Med. 2016, 9, 19185–19194. [Google Scholar]
- Qin, T.; Du, R.; Huang, F.; Yin, S.; Yang, J.; Qin, S.; Cao, W. Sinomenine activation of Nrf2 signaling prevents hyperactive inflammation and kidney injury in a mouse model of obstructive nephropathy. Free Radic. Biol. Med. 2016, 92, 90–99. [Google Scholar] [CrossRef]
- Qin, T.; Yin, S.; Yang, J.; Zhang, Q.; Liu, Y.; Huang, F.; Cao, W. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFbeta signaling. Toxicol. Appl. Pharmacol. 2016, 304, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J. Sinomenine alleviates glomerular endothelial permeability by activating the C/EBP-α/claudin-5 signaling pathway. Hum. Cell 2022, 35, 1453–1463. [Google Scholar] [CrossRef]
- Gu, H.; Li, J.; Ni, Y. Sinomenine improves renal fibrosis by regulating mesenchymal stem cell-derived exosomes and affecting autophagy levels. Environ. Toxicol. 2023, 38, 2524–2537. [Google Scholar] [CrossRef] [PubMed]
- Potocnjak, I.; Simic, L.; Baticic, L.; Krizan, H.; Domitrovic, R. Sinomenine mitigates cisplatin-induced kidney injury by targeting multiple signaling pathways. Food Chem. Toxicol. 2023, 171, 113538. [Google Scholar] [CrossRef]
- He, L.G.; Li, X.L.; Zeng, X.Z.; Duan, H.; Wang, S.; Lei, L.S.; Li, X.J.; Liu, S.W. Sinomenine induces apoptosis in RAW 264.7 cell-derived osteoclasts in vitro via caspase-3 activation. Acta Pharmacol. Sin. 2014, 35, 203–210. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Duan, H.; Li, X.; Wang, S.; Zhang, Y.; Lei, L.; Xu, J.; Liu, S.; Li, X. Sinomenine down-regulates TLR4/TRAF6 expression and attenuates lipopolysaccharide-induced osteoclastogenesis and osteolysis. Eur. J. Pharmacol. 2016, 779, 66–79. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, X.; Tang, Z.; Liu, D.; Zhou, Y.; Zeng, P.; Xiong, H. Influence of sinomenine upon mesenchymal stem cells in osteoclastogenesis. Biomed. Pharmacother. 2017, 90, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Yuan, F.; Li, Z.; Huang, S.; Shen, H.; Yuan, B. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol. Immunol. 2014, 60, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, Y.; Guan, H.; Cui, C.; Tian, S.; Yang, D.; Wang, X.; Zhang, S.; Wang, L.; Jiang, H. Effect of sinomenine on vascular smooth muscle cell dedifferentiation and neointima formation after vascular injury in mice. Mol. Cell Biochem. 2013, 373, 53–62. [Google Scholar] [CrossRef]
- Yin, Q.; Xia, Y.; Wang, G. Sinomenine alleviates high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the activation of RhoA/ROCK signaling pathway. Biochem. Biophys. Res. Commun. 2016, 477, 881–886. [Google Scholar] [CrossRef]
- Jiang, C.; Tong, Y.L.; Zhang, D.; Liu, L.Z.; Wang, J.F. Sinomenine prevents the development of cardiomyopathy in diabetic rats by inhibiting inflammatory responses and blocking activation of NF-kappaB. Gen. Physiol. Biophys. 2017, 36, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhao, B.; Jia, H.; Zhang, C.; Zuo, X. Sinomenine ameliorates cardiac hypertrophy by activating Nrf2/ARE signaling pathway. Bioengineered 2021, 12, 12778–12788. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, P.; Chen, J.; Zhang, C.; Tao, H. Protective effect of sinomenine on isoproterenol-induced cardiac hypertrophy in mice. J. Appl. Biomed. 2021, 19, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Guo, X.; He, X.; Chang, Y.; Zheng, F.; Xu, C.; Zhang, S.; Zhou, Y.; Li, J. Cardioprotective effects of sinomenine in myocardial ischemia/reperfusion injury in a rat model. Saudi. Pharm. J. 2022, 30, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Takano, F.; Yoshida, K.; Hojo, H. Protection by sinomenine against endotoxin-induced fulminant hepatitis in galactosamine-sensitized mice. Biochem. Pharmacol. 1994, 48, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Jiao, F.Z.; Yang, F.; Li, X.; Wang, L.W. Sinomenine Attenuates Acetaminophen-Induced Acute Liver Injury by Decreasing Oxidative Stress and Inflammatory Response via Regulating TGF-beta/Smad Pathway in vitro and in vivo. Drug Des. Dev. Ther. 2020, 14, 2393–2403. [Google Scholar] [CrossRef]
- Hui, B.; Shu, Y.; Yang, D.; Wang, Z.; Zhang, L.; Lei, N.; Yang, Z. Sinomenine pretreatment alleviates hepatic ischemia/reperfusion injury through activating Nrf-2/HO-1 pathway. Immun. Inflamm. Dis. 2022, 10, e700. [Google Scholar] [CrossRef]
- Li, Y.; Cai, W.; Ai, Z.; Xue, C.; Cao, R.; Dong, N. Protective effects of sinomenine hydrochloride on lead-induced oxidative stress, inflammation, and apoptosis in mouse liver. Environ. Sci. Pollut. Res. Int. 2023, 30, 7510–7521. [Google Scholar] [CrossRef]
- Liu, S.Z.; Chen, Q.H.; Liu, J.J.; Yang, X.T.; Zhang, Y.; Huang, F.J. Sinomenine protects against E.coli-induced acute lung injury in mice through Nrf2-NF-kappa B pathway. Biomed. Pharmacother. 2018, 107, 696–702. [Google Scholar] [CrossRef]
- He, H.; Cao, L.; Wang, Z.; Wang, Z.; Miao, J.; Li, X.M.; Miao, M. Sinomenine Relieves Airway Remodeling By Inhibiting Epithelial-Mesenchymal Transition Through Downregulating TGF-beta1 and Smad3 Expression In Vitro and In Vivo. Front. Immunol. 2021, 12, 736479. [Google Scholar] [CrossRef]
- Ma, J.L.; Ji, K.; Shi, L.Q.; Li, N.N.; Wang, L.Y.; Dong, S.J.; Zhang, Y.X.; Wen, S.H.; Liu, X.M.; Wang, Y.; et al. Sinomenine Attenuated Capsaicin-Induced Increase in Cough Sensitivity in Guinea Pigs by Inhibiting SOX5/TRPV1 Axis and Inflammatory Response. Front. Physiol. 2021, 12, 629276. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shu, Q.; Guan, X.; Zhao, J.; Yan, J.; Li, X.; Liu, J.; Jia, Z.; Shi, J.; Li, J. Sinomenine Protects PC12 Neuronal Cells against H2O2-induced Cytotoxicity and Oxidative Stress via a ROS-dependent Up-regulation of Endogenous Antioxidant System. Cell Mol. Neurobiol. 2017, 37, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, H.; Zhang, H. Protective effect of sinomenine against inflammation and oxidative stress in gestational diabetes mellitus in female rats via TLR4/MyD88/NF-kappaB signaling pathway. J. Food Biochem. 2021, 45, e13952. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Xia, H.M.; Li, Y.F.; Cheng, Y.F.; Wang, Y.; Xia, Y.; Yue, Y.; Cheng, X.M.; Chu, Z.X. In vitro and Ex vivo Antioxidant Activity and Sustained Release Properties of Sinomenine-Loaded Liposomes-in-Hydrogel Biomaterials Simulating Cells-in-Extracellular Matrix. Nat. Prod. Commun. 2022, 17, 1934578X2211306. [Google Scholar] [CrossRef]
- Yao, Y.M.; Cao, W.; Cao, Y.J.; Cheng, Z.N.; Ou-Yang, D.S.; Liu, Z.Q.; Zhou, H.H. Effect of sinomenine on human cytochrome P450 activity. Clin. Chim. Acta 2007, 379, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, W.; Viljoen, A.; Hamman, J.H. Effect of sinomenine on the in vitro intestinal epithelial transport of selected compounds. Phytother. Res. 2010, 24, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Z.; Tian, Y.; Liu, Z.; Wang, Q. A novel perspective and approach to intestinal octreotide absorption: Sinomenine-mediated reversible tight junction opening and its molecular mechanism. Int. J. Mol. Sci. 2013, 14, 12873–12892. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Tu, Y.; Masaki, H.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Plant-derived alkaloid sinomenine potentiates glucocorticoid pharmacodynamics in mitogen-activated human peripheral blood mononuclear cells by regulating the translocation of glucocorticoid receptor. Phytother. Res. 2019, 33, 187–196. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zou, J.; Yang, Y.; Han, R.; Zhang, J. Sinomenine Inhibits Orthodontic Tooth Movement and Root Resorption in Rats and Enhances Osteogenic Differentiation of PDLSCs. Drug Des. Dev. Ther. 2022, 16, 2949–2965. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, C.; Han, T.; Chen, J.; Ali Raza, M.; Li, B.; Wang, L.; Yan, H. Sinomenine promotes flap survival by upregulating eNOS and eNOS-mediated autophagy via PI3K/AKT pathway. Int. Immunopharmacol. 2023, 116, 109752. [Google Scholar] [CrossRef]
- Fan, M.S.; Xia, Y.F.; Ye, R.H.; Sun, Z.R.; Wang, M.Y.; An, M.F.; Zhang, S.S.; Zhang, L.J.; Zhao, Y.L.; Xiang, Z.M.; et al. Sinomenine Hydrochloride Can Ameliorate Benign Prostatic Hyperplasia by Lowering the 5α-Reductase 2 Level and Regulating the Balance between the Proliferation and Apoptosis of Cells. Molecules 2023, 28, 803. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-C.; Bi, E.-G.; Lou, Y.-T.; Wu, X.-D.; Liu, Z.-D.; Zhou, J.; Wang, Y.; Ma, Z.; Lin, G.-M.; Sun, S.-H.; et al. Novel sinomenine derivative 1032 improves immune suppression in experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 2010, 391, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liu, B.R.; Wang, J.R.; Chen, C.K.; Qin, G.W.; Lee, S.S. Two new morphinane alkaloids from Sinomenium acutum. J. Asian Nat. Prod. Res. 2011, 13, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Yuan, J.; Li, X.; Fang, N.; Lin, M.; Hou, Q.; Ji, T. Design, synthesis, and pharmacological evaluation of sinomenine derivatives on rings A and C: Novel compounds screening for aplastic anemia targeting on cytotoxic T lymphocyte. Eur. J. Med. Chem. 2021, 225, 113791. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Liu, Y.Q.; Man, J.Y.; Li, W.; Xue, S.S.; Lu, T.H.; Su, Z.L.; Zhou, C.L. C16, a novel sinomenine derivatives, promoted macrophage reprogramming toward M2-like phenotype and protected mice from endotoxemia. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211026786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, S.; Dai, Y.; Wu, L.; Jin, M.; Zhao, J.; Li, Y.; Tang, L. Sinomenine attenuated dextran sulfate sodium-induced inflammatory responses by promoting 14-3-3theta protein and inhibiting NF-kappaB signaling. J. Ethnopharmacol. 2023, 303, 116037. [Google Scholar] [CrossRef]
- Ha, J.; Park, H.; Park, J.; Park, S.B. Recent advances in identifying protein targets in drug discovery. Cell Chem. Biol. 2021, 28, 394–423. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Murata, A.; Shirakawa, T.; Uesugi, M. Biochemical target isolation for novices: Affinity-based strategies. Chem. Biol. 2010, 17, 616–623. [Google Scholar] [CrossRef]
- Lomenick, B.; Jung, G.; Wohlschlegel, J.A.; Huang, J. Target Identification Using Drug Affinity Responsive Target Stability (DARTS). Curr. Protoc. Chem. Biolog. 2011, 3, 163–180. [Google Scholar] [CrossRef]
- Hou, W.; Dai, W.; Huang, H.; Liu, S.-L.; Liu, J.; Huang, L.-J.; Huang, X.-H.; Zeng, J.-L.; Gan, Z.-W.; Zhang, Z.-Y.; et al. Pharmacological activity and mechanism of pyrazines. Eur. J. Med. Chem. 2023, 258, 115544. [Google Scholar] [CrossRef]
- Tang, J.; Raza, A.; Chen, J.; Xu, H. A Systematic Review on the Sinomenine Derivatives. Mini Rev. Med. Chem. 2018, 18, 906–917. [Google Scholar] [CrossRef]
- Chen, X.; Lu, C.; Duan, Y.; Huang, Y. Recent Advancements in Drug Delivery of Sinomenine, A Disease-Modifying Anti-Rheumatic Drug. Pharmaceutics 2022, 14, 2820. [Google Scholar] [CrossRef]
- Sun, X.Y.; Jia, L.Y.; Rong, Z.; Zhou, X.; Cao, L.Q.; Li, A.H.; Guo, M.; Jin, J.; Wang, Y.D.; Huang, L.; et al. Research Advances on Matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]










| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Cytotoxicity | IL-1β-activated Hs701.T (IC50 = 0.125 mM) | ↓: JAK3, EDG4, IL-13, PCTAIRE-3, ERF-1, HHR6A, HSP27, Daxx, TNF-A, COL1A2, IL-6, SATB, IFITM1, TNFRII, JAG2, MMP-13, and PLG. | NR | 2006 | [19] | |
| Invasion and migration inhibition | THP-1 (effect was notable at 0.05 and 1mM concentrations) | ↓: CD147, MMP-2, and MMP-9. | NR | 2009 | [20] | |
| Cytotoxicity | NCI-H460 (inhibition rate was 85.89% at 607.2 μM) | ↑: Caspase-3/-9, depolarized cells, ΔΨm disruption, cytoplasm cytochrome c, and Bax/BcL-2 ratio. | NR | 2010 | [21] | |
| Cytotoxicity | PC-3 and DU-145 (IC50 was 121.4 μM for both cell lines) | ↓: PGE, COX-2, NF-κB, andp-NF-κB (p65). | NR | 2011 | [22] | |
| Cytotoxicity | NCI-H460 (607.1 μM) | ↓: AKT and ERK1/2. | NR | 2012 | [23] | |
| Cytotoxicity Invasion and migration inhibition | MDA-MB-231 and 4T1 (SIN displayed cytotoxicity at 1 mM and showed invasion and migration inhibition at 0.25 and 0.5 mM) | ↑: CUEDC2. ↓: NF-κB binding to IκB, nuclear translocation of NF-κB, vimentin, tendine-C, CCK, MCP-1, IL-11, NF-κB activation, and p-IKK, IL-4/miR-324-5p. | NR | 2015 | [24] | |
| Cytotoxicity | A549 (0.25 mM of SIN led to apoptosis) | ↑: E-cadherin. ↓: JAK2, STAT3, p-STAT3, Snail, N-cadherin, and vimentin. | NR | 2016 | [25] | |
| Antitumor (invasion and metastasis inhibition) | HOS and U2OS cells (50–400 µM concentrations were selected for both cell lines) | ↑: TIMP-1 and TIMP-2 ↓: CXCR4, p-STAT3, VEGF, CD147, MMP-2, MMP-9, VEGF, RANKL, and p-NF-κB (p65) expression. | 150 mg/kg | ↓: RANKL-mediated osteolysis, cortical bone destruction, and number of osteoclasts. | 2016 | [26] |
| Antitumor | U87 and SF 767 (0.125–0.5 mM concentrations were selected for both cell lines) | ↓: Akt-mTOR. ↑: JNK, EB, and lysosome. | 75, 150 mg/kg | ↑: Cathepsin B/D. ↓: Tumor volume and weight, p62. | 2017 | [27] |
| Antitumor | B16-F10 (25–100 mM) | ↑: Beclin l, Bax, caspase-3, and LC3II/LC3I ratio. ↓: p-p62/SQSTML, PI3K/Akt/mTOR, and BcL-2. | 100 mg/kg | ↓: Tumor volume and weight, Ki67, and PCN. | 2018 | [28] |
| Antitumor | U87 and U251 (16 mM for both cell lines) | ↑: p53 expression. ↓: SIRT1 expression. | 100 mg/kg | ↓: U87 transplanted tumors growth. | 2018 | [29] |
| Antitumor | MDA-MB-231 (0.5 mM) | ↓: MMP-2, vimentin, IL-11, NF-κB, and it-mediated Shh pathways. | 15 mg/kg | ↑: Survival time of mice with lung metastatic breast cancer. ↓: Lung metastasis of breast cancer. | 2018 | [30] |
| Cytotoxicity | MDA-MB-231 and MCF-7 (1–16 μM concentrations for both cell lines) | ↑: p16, cleaved caspase-3/-9, PDCD-4, and miR-29. ↓: PCNA, Cyclin D1, CDK4, p-JNK, and p-MEK. | NR | 2019 | [31] | |
| Cytotoxicity | MKN45 and SGC7901 (20 μM) | ↑: Bax, cleaved caspase-3, MMP-9, vimentin, AMPK, Wnt/β-catenin, and miR-204. ↓: Cyclin D1 and BcL-2. | NR | 2019 | [32] | |
| Antitumor | NSCLC (25–100 μM concentrations were selected, but IC50 value was not reported) | ↓: p-Histone H3 (Ser10), Akt, and downstream kinase S6, HK. | 40 mg/kg | ↓: Tumor volume and weight. | 2020 | [33] |
| Cytotoxicity | Hep3B and HepG2 (2 and 4 mM for both cell lines) | ↓: p-AMPK, p-STAT3, and MARCH. | NR | 2021 | [34] | |
| Cytotoxicity | MDA-MB-231 SP (0.2–1 mM) | ↓: N-cadherin, vimentin, and MMP-2, MMP-9, p-PI3K, p-Akt, and p-mTOR. | NR | 2021 | [35] | |
| Cytotoxicity | HeyA8 (IC50 = 1.56 mM) | ↓: CDK1, p-CDK (Thr161), and p-Histone H3 (Ser10). | NR | 2021 | [36] | |
| Antitumor | SK-Hep1 (0.125–1 mM) | ↑: Cleaved caspase-9 and cleaved caspase-3. ↓: I3K/AKT1 pathway, PI3K, p85α, AKT1, BcL-2, pro-caspase-9, and pro-caspase-3. | 75, 150 mg/kg | ↓: Tumor volume and weight. | 2022 | [38] |
| Antitumor | HeLa (0.25–1 mM) | ↑: Caspase-3. ↓: Cells activity. | 70, 140 mg/kg | ↑: Tumor cell apoptosis. ↓: Tumor growth, activity of TrxR, and ROS. | 2022 | [39] |
| Cytotoxicity | Breast cancer SP cells (0.75 mM) | ↑: MiR-340-5P. ↓: SIAH2/HIF-1α pathway and epithelial interstitial transformation. | NR | 2022 | [40] | |
| Antitumor | HT-29, HCT-116, and SW-480 (2.5 mM for these three cell lines) | ↓: IL-1β and TNF-α at mRNA and protein levels; ↑: CPT1A and LPCAT3. | 120 mg/kg | ↓: Rectal neoplasia production, length of colon, number and volume of tumors, colonic mucosal injury, necrosis, submucosal edema and inflammatory cell infiltration improvement, and colitis-related tumor. | 2022 | [41] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Antitumor | Hep3B and SMMC7721 (0.5–2 µM for both cell lines) | ↑: p21, cytoplasm of Cyt c and Omi/HtrA2. ↓: ∆ψm destruction, BcL-2/Bax ratio, caspase-3/-8/-9/-10, and survivin. | 50, 100, 150 mg/kg | ↑: Apoptotic cell number. ↓: Tumor weight and volume. | 2013 | [42] |
| Antitumor | MDA-MB-231 and MCF-7 (IC50 values were 1.33 and 1.51 mM, respectively) | ↑: p21, p2, cytochrome c in the cytoplasm, cleaved PARP, Bax/BcL-2 ratios, MAPK activation, p-ERK, p-JNK, p-p38, and ROS. ↓: Cyclin D1, cyclin E, CDK4, MCM7, p-Rb, and ATM/ATR-Chk1/Chk2. | 75, 150 mg/kg | ↑: Bax/BcL-2 ratio. ↓: Tumor volume and weight, tumor proliferation marker PCNA production. | 2014 | [43] |
| Cytotoxicity | ACHN and 786-O (20 μM and 80 μM for both cell lines) | ↓: MT and EMT-related transcription factors, MMP 2, MMP 9, nail, and Twist. | NR | 2017 | [44] | |
| Antitumor | U87 and SF767 (0.0625–0.25 mM for both cell line) | ↑: p27, p21, PERK, eIF2α, IRE1α, CCAA, ER stress, and autophagy. ↓: Cyclin D1/D3/E, CDK4, free Ca2+, Vimentin, Snail, Slug, NF-κB activation, and MMP-2/-9. | 75 mg/kg | ↓: Tumor growth. | 2018 | [45] |
| Cytotoxicity | MDA-MB-231 (15.2 mM) | ↓: Cells growth and bacterial growth. | NR | 2018 | [46] | |
| Antitumor | HeLa (1 mM) | ↑: DNA damage, Chk1 activity, and cell cycle checkpoint. ↓: DDR factors KU80 and RAD51 expression. | 100 mg/kg | ↓: Tumor growth. | 2018 | [47] |
| Antitumor | Eca109, EC9706 (IC50 values were 0.3 and 0.4 mM for Eca109 and EC9706 cell lines, respectively) | ↑: Bax. ↓: BcL-2, cyclin B1, CDK1, Ku86, Ku70, and Rad5. | 75 mg/kg | ↓: Tumor growth. | 2018 | [48] |
| Cytotoxicity | SK-Hep 1 (0.25 m) | ↑: CXCL12, CXCR4, CCR7, and CCL21. ↓: ERK1/2/MMP-2/-9 signaling pathway. | NR | 2022 | [49] | |
| Cytotoxicity | BCPAP and PTC-1 (4 mM concentration was selected for both cell lines) | ↑: Thyroid iodine-processing genes, NIS, TSHR/cAMP signaling pathway, and RAI uptake. ↓: PTC cell proliferation. | NR | 2022 | [50] | |
| Antitumor | H1819 (50 µM) and H1975 (200 µM) | ↑: p-AMPK. ↓: p-mTOR. | 25, 50, 100 mg/kg | Comparable to that of cisplatin group, but toxicity was lower. | 2023 | [51] |
| Combined Drugs | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Aclarubicin | HL-60 (15.2–60.7 Nm of SIN) | ↑: Caspases-3/-9. ↓: PGE, PGE2, COX-2, and NF-κ. | NR | 2011 | [52] | |
| 5-FU | MKN-28, SGC-709, BGC-823 and HGC-27 (20–80 µM of SIN for these four cell lines) | ↑: Transfer of cytochrome c from mitochondria to cytoplasm, caspase-3/-9. ↓: TS mRNA levels. | 10 mg/kg | ↓: Tumor volume and weight in combination group. | 2013 | [53] |
| 5-FU | HepG2 (3.9 mM of SIN combined with 44.92 mM of 5-FU) | ↓: Cell activity. | NR | 2021 | [54] | |
| Adriamycin | Caco-2 and MDR-Caco-2 (500 mM of SIN for both cell lines) | ↓: PGE2, P-gp/MDR1, COX-2, and NF-κB. | NR | 2014 | [56] | |
| Cisplatin | A549 (50 μM of SIN combined with 3372.5 mM of Cisplatin) | ↑: miR-200a-3p. ↓: Glutamine metabolism. | NR | 2022 | [57] | |
| Tacrolimus and mycophenolic acid | PBMC (10–1000 μM of SIN) | ↓: Thymidine incorporation, interleukin-2 synthesis, and T lymphocyte cell cycle progression. | NR | 1999 | [58] | |
| MTX | RA-FLS (303.6 μM of SIN) | ↑: OPG and ratio of OPG/RANKL. ↓: RANKL, OPN, IL-6, IL-17, MMP-1, and MMP-3/-13. | 120 mg/kg | ↓: Synovial inflammation and joint injury. | 2014 | [59] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-inflammatory | NR | 150 mg/kg | Joint swelling and ESR. | 1996 | [71] | |
| Anti-inflammatory | PMs and synoviocytes (91.1–364.3 μM for both cell lines) | ↑: IκBα. ↓: TNF-α, IL-1β, and NF-κB. | NR | 2005 | [72] | |
| Anti-angiogenic | HUVEC (125–1000 μM) | NR | NR | 2005 | [73] | |
| Anti-inflammatory | PMs and synoviocytes (276.5–1105.9 μM for both cell lines) | ↑: IκBα. ↓: TNF-α, IL-1β, and NF-κB. | NR | 2005 | [74] | |
| Anti-colitis | NR | ↓: TNF-α and IFN-γ. | 100, 200 mg/kg | ↑: Myeloperoxidase activity. ↓: Body weight, macroscopic score, and histological score. | 2007 | [77] |
| Inhibited activation of retinal microglia cells | Retinal microglia cells (0.1 mM and 1 mM y) | ↓: TNF-α, IL-1β, IL-6, ROS, and nuclear translocation of NF-κB p65. | NR | 2007 | [78] | |
| Analgesic | CHO cells (1 μM and 10 μM) | ↑: p-OMR. | 10, 20, 30 mg/kg | ↑: OMR activation. | 2008 | [79] |
| Anti-OA | NR | ↑: TIMP-1/-3. ↓: IL-1β, IL-6, and MMP-2/-9. | 100 mg/kg | ↓: Incidence and progression of CIA, foot swelling, ESR, and arthritis score. | 2008 | [80] |
| Anti-MSPGN | NR | ↓: T-bet, T-bet/GATA-3 ratio, and IFN-γ. | 240 mg/d | ↓: Albuminuria. ↑: Complement C3. | 2009 | [81] |
| Anti-OA | SW1353 and human osteoarthritic chondrocytes (1–5 mM for both cell lines) | ↓: MMP-1, MMP-3, MMP-9, and MMP-13, catabolism of IL-1β, and proteolytic enzymes. | NR | 2010 | [82] | |
| Anti-OA | Chondrocytes (10–250 mM) | ↑: TIMP-1. ↓: IL-1, β-induced GAG, and MMP-13. | NR | 2010 | [83] | |
| Anti-inflammatory | FLS and THP-1 (0.01–1.00 mM for both cell lines) | ↓: Invasion and migration ability, CD147, and MMP-2/-9. | NR | 2011 | [84] | |
| Anti-inflammatory | RA-FLS (75.9–607.2 μM) | ↓: VCAM-1, IL-6, CCL 2, CXCL8, p-IκBα, and NF-κB. | NR | 2011 | [85] | |
| Anti-inflammatory | HMC-1 (IC50 = 52.73 μM) | ↓: TNF-α, IL-6, IL-8, COX-2, p-ERK1/2, p-p38 MAPK, p-κBα, and NF-κB. | NR | 2012 | [86] | |
| Anti-RA | RAW264.7 (0.0625–1 mM) | ↓: c-Src, MMP-9, TRACP, TRAF6, NF-κB, IκBα degradation and translocation of p65 to the nucleus, p-p38 and p-JNK, Ca2+ influx, NFATc1, AP-1, Fra-1, Fra-2, and c-Fos. | 80 mg/kg | ↑: Body weight. ↓: Hind paw swelling and bone loss. | 2013 | [87] |
| Anti-colitis | NR | ↓: MPO activity, miR-155, c-Maf, TNF-α, and IFN-c. | 100, 200 mg/kg) | ↑: Weight and survival rate, colon symptoms, and histological scores. ↓: Diarrhea score. | 2013 | [88] |
| Anti-RA | NR | ↓: TNF-α, IL-1β, and IL-6. | 100 mg/kg | ↓: Synovial hypertrophy, cartilage damage, joint space narrowing, osteoporosis, cartilage, and bone erosion. | 2013 | [89] |
| Analgesic | NR | GABAA | 10–40 mg/kg | ↑: Paw withdrawal threshold. ↓: Duration of immobile behavior, depression-like behavior, and chronic pain. | 2014 | [90] |
| Anti-macrophage activation | RAW264.7 (100 μM) | ↓: TNF-α, IL-6, α7nAChR, and NF-κB p65. ↑: Cytoplasmic IκBα. | NR | 2015 | [91] | |
| Anti-arthritic | RA-FLS (0.125–1 mM) | ↓: ALP activity, MyD88, and TRAF-6. | NR | 2015 | [92] | |
| Analgesic | NR | GABAA. | 5–80 mg/kg | Analgesic effect on postoperative rats via GABAA receptor. | 2016 | [93] |
| Anti-inflammatory | NR | TGF-β1/CTGF pathway and oxidative stress. | 25, 50, 75 mg/kg | Asthmatic mice airway inflammation and remodeling alleviation | 2016 | [94] |
| Anti-arthritic | Treg cells (0.1–1 mM) | ↑: IL-10 level. ↓: Foxp3, IL-10, RoRγT, IL-17a, IL-17f, IL-21levels, Th17 cells, Treg cells, L–1β, TNF-α, IL-6, and IL-17. | 120 mg/kg | ↓: Arthritis index, inflammation and cartilage damage, and paw swelling. | 2016 | [95] |
| Anti-neuropathic pain | HEK293 (10 μM) | ↓: ATP activation, P2X3, p-P38MAPK, and pain behavior alleviation. | 40 mg/kg | MWT (about 50%) and TWL (about 80%) enhancement | 2017 | [96] |
| Anti-inflammatory bowel disease | NR | ↑: SIGIRR and IL-10. ↓: TLR/NF-κB, IFN-γ, IL-1β, TNF-α, IL-12p70, and IL-6. | 30, 90, 270 mg/kg of SIN; 180, 540, 1600 mg/kg of SIN microspheres | The colon length of SIN microspheres group was longer than SIN group; histological grade score of SIN microspheres group was lower than SIN group. | 2017 | [97] |
| Anti-endotoxin | Endothelial cells (3 mM) | ↓: Key control genes in the pathogenesis of LPS. | NR | 2018 | [98] | |
| Anti-microglial inflammatory response | BV-2 (25–100 μM) | ↑: IκB-α and miRNA-183-5p. ↓: SP1/miRNA-183-5p/IκB-α pathway, p-p65, p-p50, TNF-α, IL-1β, IL-6, and SP1. | NR | 2018 | [99] | |
| Anti-RA | RAW264.7 (3–151.8 µM) | ↓: IL-6, GM-CSF, IL-12p40, IL-1α, TNF-α, IL-1β, KC (CXCL1), Eotaxin-2, IL-10, M-CSF, RANTES, and MCP-1. | 50, 100 mg/kg | ↓: Swollen paw score, inflammation score, and cartilage damage score of CIA mice, weight loss. | 2018 | [100] |
| Anti-arthritis | PC12 (0.03–0.3 mM) | ↑: α7nAChR-PI3K/Akt/mTO. | 120 mg/kg | VIP production promotion in the gut and neuronal cells. | 2018 | [101] |
| Anti-inflammatory pain | NR | ↓: P38MAPK, NF-κB, TNF-α, IL-1b, IL-6, p-p65, p-p3, COX-2, and PGE2. | 30 mg/kg | ↓: Inflammatory pain. | 2018 | [102] |
| Anti-inflammation of eye tissue | NR | ↓: NF-κB, TNF-α, PG-E2, and translocation of NF-κB p65 subunits to the nucleus. | 50, 100 mg/kg | ↓: Number of inflammatory cells, protein leakage. | 2018 | [103] |
| Anti-arthritis | NR | ↑: OD and MDA. ↓: NF-κB and MAPK, TNF-α, IL-6, IL-1β, IL-8, COX-2, iNOS, and MMP-2/-9. | 50 mg/kg | ↑: Total body weight of the rat. ↓: Paw volume and arthritis score. | 2018 | [104] |
| Anti-colitis | NR | ↑: Nrf2/NQO-1 and SOD activity. ↓: TNF-α, IL-6, and iNOS level. | 100 mg/kg | ↓: Body weight and DAI score, colon shortening, and colitis histological damage. | 2018 | [105] |
| Anti-inflammatory | MG63 (0.25–1 mM) | ↑: SOD, CAT, Nrf2, HO-1, NQO-1, and Nrf2. ↓: MAPKP38/NF-κB, IL-1β, IL-6, TNF-α, p-p38, p-NF-κB (P65), and MDA. | NR | 2018 | [106] | |
| Analgesic | NR | ↓: JAK2/STAT3 and CAMKII/CREB. | 10, 20, 40 mg/kg | Mechanical hypersensitivity of pain in cancer bone algia rat alleviation, and microglia activation inhibition. | 2018 | [107] |
| Anti-RA | NR | ↑: IL-10. ↓: IL-1β, IL-6, and TNF-α. | 1.5 g/kg | The extract (4:1, 1.5 g/kg) and extract (3:1, 1.5 g/kg) groups were superior to SIN. | 2018 | [108] |
| Anti-OA | Mouse chondrocytes (6.25–25 μM) | ↑: Nrf2/HO-1. ↓: NF-κB, iNOS, COX-2, NO, PGE2, TNF-α, IL-6, P-NF-κB p65, p-IκBα, ADAMTS-5, and MMP. | 10 mg/kg | ↑: Thickness of articular cartilage. ↓: Degradation of ECM. | 2019 | [109] |
| Anti-OA | Mouse chondrocyte (30 μM) | ↓: IL-6, TNF-α, MiR-192, NF-κB, and MAPK. | NR | 2019 | [110] | |
| Anti-RA | NR | ↓: TNF-α, IL-6, ROS, and ESR. | 5 mg/kg (AS-TE) | ↓: Joint swelling, bone defects. | 2019 | [111] |
| Anti-inflammatory | HaCaT (1 μM) | ↓: IL-6, TNF-α, COX-2, iNOS, p-P65, p-IκBα, p-p38-MAPK, NF-κB, MAPK, and CAT1. | NR | 2019 | [112] | |
| Anti- inflammatory | NR | ↓: α7nAChR. | 120 mg/kg | ↓: Paw swelling, AI, TNF-α, and ESR. | 2019 | [113] |
| Anti-inflammatory | Raw264.7 (300 μM) | ↑: p-STAT3 and JAK2/STAT3. ↓: TNF-α, MCP-1, MIF, MMP-9, CD14, TLR4, intracellular Ca2+, and NF-κB. | NR | 2019 | [114] | |
| Analgesic | NR | 0–80 mg/kg | Analgesic effect of older pups was better than that of younger pups. | 2020 | [115] | |
| Anti-inflammatory | Macrophage (303.6–3036 μM) | ↓: TNF-α, IL-1β, IL-6, TLR4, MyD 88, p-IκB, macrophage immune response, and TLR4/NF-κB. | NR | 2020 | [116] | |
| Anti-inflammatory | Raw264.7 (3–75.9 μM) | ↑: SOCS1. ↓: TNF-α, IL-1β, IL-6, inflammatory responses, miR-155, and NF-κB. | NR | 2020 | [117] | |
| Anti-rheumatoid arthritis | NR | 2 mg/kg | Target RA site. The leakage of SIN prevention. | 2020 | [118] | |
| Promote MMP production | SW1353 (25–100 µM) | ↑: SOCS3. ↓: MMP, p-JAK2, and p-STAT3. | NR | 2020 | [119] | |
| Anti-inflammatory | Raw264.7 (10–200 μM) | ↑: IL-6, TNF-α, and NF-κB nuclear translocation. ↓: ROS and LDH. | NR | 2020 | [120] | |
| Anti-inflammation of dorsal root ganglion | DRG (800 μM) | ↓: P38MAPK, CREB, c-fos, p-CAMKII, NF-κB, COX2, TLR4, IL-1B, IL-17A, and p38MAPK/CREB. | 20 mg/kg | ↓: MWT and TWL. | 2021 | [121] |
| Anti-RA | PBMC (0.3–30 μM) | No direct effect on T cells. | NR | 2021 | [122] | |
| Anti-foodborne enteritis of fish | NR | ↓: TNF-α, IL-10, IL-22, and FOXP3a. | 35 ppm | ↑: Intestinal villus height ↓: Inflammation and dysregulation. | 2021 | [123] |
| Anti-neuropathic pain | NR | ↓: NF-α, IL-1β, IL-6; RIP3, p-JNK, c-Fos, and IP3/JNK. | 20, 40mg/kg | ↑: Survival neurons of spinal dorsal horn. | 2021 | [124] |
| Anti-migration and anti-inflammatory | RAW264.7 and BMDMs (160–640 μM for both cell lines) | ↓: RC/FAK/P130CAS, iNOS/NO, integrin αV, integrin β3, TNF-α, and IL-6. | 25, 50, 100 mg/kg | ↓: Migration of Mouse mononuclear macrophage leukemia cells Raw264.7 to the foot and swelling of the foot. | 2021 | [125] |
| Anti-arthritic | RASFs (12.5–100 μM) | ↑: Degradation of Keap1, HO-1, and p- p62 (Thr269/Ser272). ↓: IL-6, IL-33, ROS, Nrf2, and p-p62 (Ser35). | 25, 50, 100 mg/kg | ↓: Incidence rate of CIA mice and swelling of the hind paws. | 2021 | [126] |
| Anti-RA | FLSs (200 μM) | ↑: A2AR and cAMP. ↓: MCP-1, IL-6, vascular endothelial growth factor, and NF-κB pathway. | 120 mg/kg | ↓: Arthritis index, the hind paw volume, ESR, and TNF-α. | 2021 | [127] |
| Anti-RA | NR | ↑: Solubility. ↓: Drugs release. | NR | 2021 | [10] | |
| Anti-colitis | NR | ↑: IL-10 and arginine 1. ↓: TNF-α, IL-6, inducable nitric oxide synthase, NOD-, LRR-, and NLRP3 inflammasome. | 100 mg/kg | Intestinal microbial composition alteration. | 2021 | [129] |
| Anti-pneumonia | WI-38 (5–20 μM) | ↓: TNF-α, IL-1β, MCP-1; IL-10, and GSTM 1. | NR | 2022 | [130] | |
| Anti-arthritis | RAW264.7 cell (50–400 µM) | ↓: TNF-α and inflammatory factors. | 25, 50, 100 mg/kg | ↓: Mean joint score and foot volume. | 2023 | [51] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-PD | Microglia and mesencephalic glial cells. (10−14 M for both cell lines) | ↓: ROS, NO, iNO, TNF-α, PGE2, COX-2, and microglial superoxide production. | NR | 2007 | [151] | |
| Neuroprotective | BV2, HT22 and primary hippocampal cells (0.4 mM for both cell lines) | ↓: ROS, NO, TNF-α, IL-6, and MCP-1. | NR | 2011 | [152] | |
| Anti-TBI | NR | ↑: BcL-2, ARE pathways, Nrf2, and GPX and SOD activities. ↓: Caspase-3 and MDA. | 10, 30, 50 mg/kg | ↑: Motor ability recovery promotion ↓: Brain edema reduction. | 2016 | [153] |
| Anti-ischemic stroke | Primary mixed glial cell (0.1–1.0 mM) | ↓: NLRP3, ASC, caspase-1, IL-1β, IL-6, IL-18, TNF-α, and OGD-induced NLRP3 inflammasome activation. | 10, 20 mg/kg | ↓: Cerebellar infarct size, brain water content, neuronal loss, and neurological deficit. | 2016 | [154] |
| Anti-ICH | Microglia cell (1 mM) | ↑: M2. ↓: Microglia migration, M1, and microglia-mediated neuronal toxicity. | 100 mg/kg | ↓: Infiltration of microglia activation. ↑: Rain water content and nerve damage and microglia M2 polarization. | 2016 | [155] |
| Hypnosis | NR | ↑: Flow of Cl− in hypothalamic neurons, glutamic acid decarboxylase (GAD 65/67), and hypothalamic GABA subunits (α4, β1, β2, γ3). | 20, 40 mg/kg | ↓: Spontaneous activity inhibition and sleep latency of pentobarbital. ↑: Total sleep time. | 2017 | [156] |
| Prevention of morphine-induced CPP | SH-SY5Y cells (100 µM) | ↓: NMDAR 1/CAMKII/CREB, cAMP and Ca2+, p-NMDAR1/NMDAR1, p-CAMKII/CAMKII, and p-CREB/CREB. | 60 mg/kg | ↓: Astrocyte activation. | 2018 | [157] |
| Anti-epileptic | NR | ↓: NLRP 1 inflammasome complex, IL-1β, IL-18, IL-6, and TNF-α. | 20, 40, 80 mg/kg | ↑: Neuroprotective effects: kindling acquisition process disruption and seizure latency. ↓: Seizure duration, spatial learning, and memory damage. | 2018 | [158] |
| SIN reduced formalin-induced injurious behavior in mice | NR | ↓: p-ERK1/2. | 80 mg/kg | ↓: Formalin-induced licking and biting responses. | 2019 | [159] |
| Anti-SCI | PC12 (10 μM) | ↑: Nuclear Nrf2, and Nrf2 nuclear translocation. ↓: IL-1β, IL-6, and TNF-α inhibition. | 40 mg/kg | ↓: Spinal cord edema. | 2019 | [160] |
| Anti-AD | C8D1A (100 μM) | ↓: ROS, NO and IL-12p70, IL-10, IL-6, IL-1β, and IL-8, and toxic factors. | NR | 2020 | [161] | |
| Effects of SIN on orphine-induced zebrafish | NR | 40, 80 mg/kg | ↑: TH and NR2B, zfmor, zfdor1, and zfdor2. ↓: CPP effect. | 2021 | [162] | |
| Anti-MS | NR | ↑: IL-10. ↓: IL-1β, IL-6, IL-18, TNF-α, and IL-17A. | 100 mg/kg | Neuroinflammation, demyelination, axon damage, and loss alleviation. | 2021 | [163] |
| Anti-brain injury | BV2 (50–200 µM) | ↑: M2 markers (Arg 1 and IL 10), Nrf2, HO1, and NQO1 ↓: TNF-α, IL-1β, NOS2, SOD, GPx, M1 markers (NOS2 and IL 6), p-IκBα, and nuclear translocation of NF-κB. | 20 mg/kg | ↓: Pathological lesion of brain tissue and water content of brain. | 2021 | [164] |
| Cognitive dysfunction promotion | NR | 100 mg/kg | ↑: Short-term Y-maze alternations, dark avoidance latency of passive avoidance pattern. ↓: Detection error and latency of Barnes maze task and cognitive dysfunction. | 2022 | [165] | |
| Anti-PD | NR | ↑: Beclin 1, LC3-II/LC3-I ratio, and LC3B-positive neurons ↓: PI3K/Akt/mTOR pathway and P62. | 20 mg/kg | Motor function of PD mice improvement and survival of dopaminergic neurons promotion. | 2022 | [167] |
| Anti-IVDD | RAW264.8 and NPCs (75.9 μg/mL for both cell lines) | ↑: ARG-1, M1 to M2, IL-10, type II collagen, SOD, and BcL-2. ↓: iNOS, TNF-α and IL-6, ROS and MDA, Bax, caspase-3, MMP-2, and MMP-9. | NR | 2022 | [166] | |
| Diabetic peripheral neuropathic pain (DPNP) alleviation | NR | ↓: PTGS2. | 1 mL/kg | ↓: Blood glucose levels and increased body weight. | 2023 | [168] |
| SAH-induced early brain injury (EBI) remission | NR | ↑: BcL-2. ↓: Bax, IL-1β, IL-6, and SAH-derived microglia. | 50, 100 mg/kg | ↓: Water content of cerebral cortex and apoptotic fraction. | 2023 | [169] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Immunosuppression | NR | 30, 100 mg/kg | ↓: Anti-SRBC PFC, number of spleen cells. | 1985 | [170] | |
| Kidney protection | TECs CD4+ T (303.6 μM) | ↑: IL-2 and IFN-γ. ↓: B7-H1 and B7-DC. | NR | 2005 | [171] | |
| Inducing CD4T cell apoptosis | CD4+ T (0.1 mM and 1 mM) | G1 phase blockade ↓: Caspase-3 cleavage. | NR | 2007 | [172] | |
| Anti-MS | NR | 50, 100, 200 mg/kg | ↓: EAE clinical scores, percentage of initial weight loss, TNF-α, IFN-g, MIP-1A, and MCP-1. | 2007 | [173] | |
| Promoting DC differentiation | DC (607.2 μM) | ↑: Differentiation of monocytes into DCS, CD1a. ↓: CD14, CD86, CD40, B7-H1, HLADR, CD32, MR reversion, IFN-γ, and IL-2 production. | NR | 2007 | [174] | |
| Anti-rheumatic heart disease | HUVECs (0.5 M and 1.0 M) | ↓: VCAM-1. | NR | 2007 | [175] | |
| Promoting DC maturation | DC (2 mM and 5 mM) | ↓: HLA-DR, CD40, CD80, CD86, CD83, CPM value, IL-1, NF-κB, p-IκBα, and migration of RelB from cytoplasm to nucleus. | NR | 2007 | [176] | |
| Promoting RBL-2H3 activation | RBL-2H3 (0.5–2 mM) | ↓: β-amino-hexosamine release, IL-4, TNF-α, p-GAB2, p-Akt, and p-p38MAPK. | NR | 2008 | [177] | |
| Inhibiting L-histidine decarboxylase | L-histidine decarboxylase (IC50 = 969 mM, KI = 762 mM) | ↓: L-histidine decarboxylase inhibition. | NR | 2009 | [178] | |
| Promote cell threshing | RBL-2H3 (31.25–2 mM) | ↑: β-hhexosaminidase, P-CPLA2, p-ERK promotion, ANXA1 cleavage, and COX-2. ↓: Degranulation of RBL-2H3 cell induction. | NR | 2015 | [179] | |
| Anti-macrophage activation | P815 (0.1–100 μM) | ↑: β-hexosaminidase release, IP3, intracellular Ca2+, IP 3R, p-Lyn, and PLCγ1. ↓: Histamine release. | 0.364, 1.82, 9.10 mg/kg | ↑: Mouse ear vascular permeability, IP3, and TNF-α. | 2016 | [180] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-depression | NR | ↑: BDNF, pTrkB, and pCREB. | 20, 40 mg/kg | ↑: Social interaction and sucrose preference. ↓: Forced swimming and tail suspension tests immobility. | 2018 | [182] |
| Anti-depression | NR | ↑: NE and 5-HT. ↓: Imbalance of hippocampal neurotransmitter, IL-1b, IL-6, TNF-α, P38-MAPK-NF-κB, NLRP 3, ASC, and caspase-1. | 30, 100, 300 mg/kg | ↑: Weight and sucrose consumption. ↓: Depressive symptoms alleviation, time of forced swimming, and tail suspension tests. | 2018 | [183] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-sepsis | PM (100 μM) | ↑: Autophagosome formation. ↓: IL-6 and TNF-α release. | 100 mg/kg | CLP-induced organ damage attenuation. | 2015 | [184] |
| Anti-ALI | RAW264.7 (25–100 μM) | ↑: Nrf2 and autophagy pathways, Nrf2, HO-1, SOD, LC-3II, ATG5, and Beclin1. ↓: KEAP1, NQO1, IL-6, TNF-α, and serum content of MD. | 100 mg/kg | ↓: LPS-induced lung injury and W/D ratio of lung tissue. | 2020 | [185] |
| Anti-septic lung injury | Caco-2 (50–400 μM) | ↑: Aromatic hydrocarbon receptor, CYP1A1, Claudin1, Nrf2, HO-1, and NQO-1. | 100 mg/kg | Intestinal microbiome regulation, intestinal barrier restoration, composition of beneficial and harmful bacteria changed, endotoxins produced prevention, inflammatory response inhibition, and acute lung injury protection. | 2021 | [186] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-kidney injury | NR | ↑: Nephrin and podocin, PPAR-α. ↓: TNF-α and IL-1β. | 10, 30 mg/kg | ↑: Total protein and serum albumin ↓: Weight loss, foot process width of rats, and urinary protein excretion cholesterol and triglycerides. | 2012 | [187] |
| Anti-kidney injury | NR | ↓: XCL-10, ICAM-1, TNF-α, IL-6, p-IKK-β, IκB-α, NF-κB, MAPk, p-P44/42, p-JNK, and p-P38. | 200 mg/kg | ↓: Serum CR and BUN, renal histological damage, and inflammatory infiltration. | 2013 | [188] |
| Anti-kidney injury | HK-2 (0.1–50 μM) | ↑: MiR-124. ↓: Apoptosis. | 200 mg/kg | ↑: SOD. ↓: MDA, MPO, inflammatory infiltration, and renal cell apoptosis induced via I/R. | 2016 | [189] |
| Anti-Kidney Injury | HEK293 (25–100 μM) | ↑: Nrf2, HO-1, NQO1, ARG-1, and IL-10. ↓: Keap1. | 100 mg/kg | ↓: Renal fibrosis, E-Cadherin, α-SMA and fibronectin, IL-1β, and TNF-α. | 2016 | [190] |
| Anti-renal fibrosis | HEK293 (25–100 μM) | ↑: Nrf2, HO-1, NQO1, and NRF2. ↓: GFβ/Smad Wnt/β-catenin, and TGFβ-induced ROS levels. | 100 mg/kg | ↓: Renal tubular dystrophy and fibrosis area. | 2016 | [191] |
| Anti-diabetic nephropathy (DN) | HrGECs induced by hyperglycemia (HG) (50 μM and 100 μM) | ↑: Activation of C/EBP-α/Claudin-5 axis. ↓: Production of IL-18 and IL-1β within cells. | 20, 40 mg/kg | ↓: Blood glucose, body weight, kidney tissue pathology, infiltrating inflammatory cell, and alleviated glomerular endothelial dysfunction. | 2022 | [192] |
| Anti-renal fibrosis | NR | ↑: PIK3CB TGF-β1, miR-204-5P, autophagy induction, and M2-type polarization. ↓: Creatinine and BUN, PI3K/Akt, pro-inflammatory cytokine, and M1-type polarization. | 50, 100 mg/kg | ↓: Body weight and kidney weight. | 2023 | [193] |
| Anti-cisplatin (CP)-induced kidney injury | HK2 (IC50 = 280 μM) | ↑: p21, Bax, Noxa, PARP1, BcL-2, and SIRT6. ↓: HO-1, 4-HNE, 3-NT, TNF-α, STAT3, p-STAT3, NF-κB p65, and caspase-3/-8. | 5 mg/kg | ↓: Blood BUN, creatinin, NGAL, KIM-1, and histopathological scores. | 2023 | [194] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Promoting osteoclast apoptosis | RAW 264.7 (0.25–2 mM) | Activating caspase-3. | NR | 2014 | [195] | |
| Anti- lipopolysaccharide-induced osteoclastogenesis and osteolysis | RAW264.7 cell (0.25–1 mM) | ↓: Tracp, MMP-9, C-src, integrin αVβ3 and CK, TNF-α production, c-fos, Fra-1 and Fra-2, intracellular Ca2+ influx, TLR4, TRAF6, MAPK (p-P38), NF-κB, AP-1, NF-ATc1, and TLR. | 25, 50, 100 mg/kg | ↓: Craniolysis and TNF-α. | 2016 | [196] |
| Regulation of MSCs | MSCs (0.25 mM) | ↑: OPG. ↓: PTGES3, PGE2, and RANKL OPG/RANKL ratio. | NR | 2017 | [197] | |
| Anti-brain injury | BV-2 microglia (0.1 mM and 1 mM) | ↓: Erythrocytolysis-induced TNF-α, IL-1, IL-6, ROS, ICH induced microglial activation, NF-B, and migration. | 20 mg/kg | ↓: Brain water content and neurological deficit score. | 2014 | [198] |
| Anti-ischemic | Astrocyte (1 mM) | ↑: CRYAB/STAT3, DRD2, CRYAB, CRYAB, and CRYAB interaction with STAT3. ↓: STAT3, p-STAT, CRYAB nuclear translocation, and STAT3 DNA-binding activity. | 10, 20 mg/kg | ↓: Ischemic infarct volume and neuronal apoptosis, neurological impairment. | 2016 | [9] |
| Anti-TBI | RAW264.8 (151.8–910.8 μM) | ↓: Early/acute inflammatory responses, TNF-α, IL-1β, CCL-3, IL-6, Oxidative stress (iNOS and NO), and NF-κB and its nuclear shift. | 30 mg/kg | Specifically targets activated microglia/macrophages. | 2020 | [199] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-vascular injury | VSMC (200 μM) | ↓: Phosphorylation of ERK1/2 and p38, Akt, GSK3β, STAT3, and PDGFR-β. | 150 mg/kg | ↓: Formation of neointima and number of PCNAP cells. | 2013 | [200] |
| Improving renal function | HRGECs (25–100 mM) | ↑: Occludin, Nr. ↓: RhoA/ROCK signal transduction activation, abnormal occlusive protein distribution reversion, RhoA/ROCK, Cell permeability, and ROS. | NR | 2016 | [201] | |
| Improving cardiac function | NR | ↓: NF-κB and cytokines, IκB expression, CD3+- and CD68+-positive cells infiltration, TNF-α, IL-1, and IL-6 level. | 30, 60, 120 mg/kg | ↑: Heart rate and EF. ↓: Cardiac function and pathological symptoms, IVSD, LVEDD, LVESD, cardiac index, and cardiomyocyte hypertrophy. | 2017 | [202] |
| Anti-CH | H9C2 cells (50–100 μM) | ↓: Cell surface area and apoptosis rate, ROS and MDA, Caspase-3, and Bax. ↑: BcL-2 and Nrf2/AR. | 40, 80 mg/kg | ↓: Heart weight and left-ventricular mass index. | 2021 | [203] |
| Anti-CH | NR | ↓: NF-κB, TNF-α, and IL-1β. | 120 mg/kg | ↑: Level of SOD. ↓: LVWI, LVAWd and LVPWd, degree of myocardial hypertrophy, inflammatory cell infiltration, interstitial fibrosis, and contents of LDH and MDA. | 2021 | [204] |
| Anti-I/R | NR | ↑: SOD, GPx and CAT. ↓: CK-MB, CK, Tnl, TXB 2, TF, Fbg, PA1-1, MDA, LDH, AST, Hs-CRP, MCP-1, NF-α, IL-1β, and IL-6. | 5, 10, 20 mg/kg | ↓: Frequency, duration, and incidence of VT and VF, incidence of VEB, and area of myocardial infarction. | 2022 | [205] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-inflammatory | NR | 10–100 mg/kg | ↓: Liver injury, TNF, and ROS. | 1994 | [206] | |
| Anti-acute liver injury | BRL-3A (30.4–303.6 μM) | ↑: SOD and GSH-Px. ↓: TGF-β/Smad, MDA, LDH d, TNF-α, IL-1β, IL-6, NLRP3, ASC, caspase-1, IL-1β, and NLRP3. | 100 mg/kg | ↓: Liver dysfunction. | 2020 | [207] |
| Anti-IR in liver | NR | ↑: TNF-α, IL-6, IL-8, IL-10, and Nrf-2/HO-1. | 100 mg/kg | ↓: ALT, AST, LDH, inflammatory cell infiltration, and proportion of cytoplasmic vacuoles and necrotic cells. | 2022 | [208] |
| Protect liver injury caused by Pb | NR | ↑: BcL-2. ↓: Caspase-3, Bax, IL-1β, TNF-α, NF-κB, NF-κB p65, and IκBα. | 100 mg/kg | ↑: T-AOC, body weight of Pb mice. ↓: Liver index, serum ALT, AST, LDH, liver lead content of Pb mice, and MDA level. | 2023 | [209] |
| Treatment of acute lung injury | NR | ↑: SOD, NQO1, Nrf2, HO-1, PKC, and Nrf2. ↓: Nrf2/Keap1/PKC, p-NF-κB p65, cytokines, NF-κB, TNF-α, IL-6, IL-1β, MDA, and Keap1. | 100 mg/kg | ↓: Lung W/D ratio, neutrophil infiltration, pulmonary edema, alveolar wall thickening and cell structure destruction, level of BALF, BALF neutrophils, and MPO. | 2018 | [210] |
| Anti-airway remodeling | 16Hbe (607.2 mM) | ↓: Cell migration, MMP7, MMP9, and vimentin. | 35, 75 mg/kg | ↓: IgE, IL-4, airway remodeling alleviation, subepithelial collagen deposition, EMT, TGF-B1, and Smad3. | 2021 | [211] |
| Anti-cough | NR | ↓: TRPVl, SOX5, intracellular Ca2+, SP, and NKA. | 5000 mg/kg | ↓: Capsaicin-induced high cough sensitivity and inflammatory cell infiltration. | 2021 | [212] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Anti-oxidative stress | PC12 (5 μM) | ↑: Nrf2 antioxidant system, cells survival rate, Nrf2, HO-1, NQO-1, and antioxidant genes expression. ↓: NOX and oxidative stress response. | NR | 2017 | [213] | |
| Anti-GDM | NR | ↓: IL-1β, TNF-α, IL-6, NF-κB, MYD88, NLRP3, TLR4, NF-κB, GDM, and TLR4. | 10 mg/kg | ↑: TAC, GST, and SOD. ↓: LPO. GPX, | 2021 | [214] |
| DPPH and H2O2 clearance | Mouse skin (DPPH: IC50 = 25.5 μM, H2O2: IC50 = 1.1 mM) | NR | 5 mM | MDA inhibition | 2022 | [215] |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| CYP2C19 inhibition | Cytochrome P450s (50 μM) | ↑: Elimination of mephentoin promotion. ↓: CYP2C19. | NR | 2007 | [216] | |
| Promoting drug absorption | Intestinal epithelial (0.5%, 1%, and 2% w/v) | ↑: Apical-to-basolateral transport, drug absorption, tight junction transients, and molecular stability. ↓: TEER, basolateral-to-apical transport of the P-gp substrate cimetidine, ability of active efflux of P-gp substrates, and active drug efflux and transport. | NR | 2010 | [217] | |
| Promoting intestinal absorption | Caco-2 cell (0.5% w/v) | ↑: FD-4 flux, intestinal OCT absorption, and PKC signaling pathway. ↓: TEER and Claudin-1. | 30 mg/kg | ↑: Pharmacokinetic behavior of OCT. Intestinal absorption of OCT. | 2013 | [218] |
| Anti-RA | Jurkat T/PBMCs (0.03–3 μM) | ↑: Jurkat T cells and normal PBMCs GR translocation regulatory. ↓: PBMCs proliferation and IC50 value of MP. | NR | 2019 | [219] | |
| Activity | Cell Type or Model In Vitro (Effective Concentrations or IC50 Values) | Mechanism of Action | In Vivo | Year | Ref. | |
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Therapeutic Effect | |||||
| Effects on teeth in rats | PDLSCs (0.1–2.0 M) | ↑: ALP activity, deposition of mineralized nodules, OPG, ALP, and RUNX2. ↓: RANKL. | 20, 40 mg/kg | ↑: Alveolar bone structure, BV/TV, OPG, RUNX2, and OCN. ↓: Trap-positive osteoclasts on the compression side, RANKL, and TNF-α. OTM and root resorption inhibition. | 2022 | [220] |
| Promoting skin flap survival | HUVECs (80 μM) | ↑: eNOS, autophagy flux, angiogenesis, cell apoptosis decrease, eNOS, flap survival, and PI3K/Akt pathway. ↓: Oxidative stress. | NR | 2023 | [221] | |
| Improving benign prostatic hyperplasia | BPH-1 (25, 50 and 100 μM) | ↑: Bax. ↓: SRD5A2, PCNA, BcL-2, and MMP2. | 0.5, 1, 2 mg/kg | Decreased prostate gland (PG) weight coefficient in BPH mice | 2023 | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Huang, L.; Huang, H.; Liu, S.; Dai, W.; Tang, J.; Chen, X.; Lu, X.; Zheng, Q.; Zhou, Z.; et al. Bioactivities and Mechanisms of Action of Sinomenine and Its Derivatives: A Comprehensive Review. Molecules 2024, 29, 540. https://doi.org/10.3390/molecules29020540
Hou W, Huang L, Huang H, Liu S, Dai W, Tang J, Chen X, Lu X, Zheng Q, Zhou Z, et al. Bioactivities and Mechanisms of Action of Sinomenine and Its Derivatives: A Comprehensive Review. Molecules. 2024; 29(2):540. https://doi.org/10.3390/molecules29020540
Chicago/Turabian StyleHou, Wen, Lejun Huang, Hao Huang, Shenglan Liu, Wei Dai, Jianhong Tang, Xiangzhao Chen, Xiaolu Lu, Qisheng Zheng, Zhinuo Zhou, and et al. 2024. "Bioactivities and Mechanisms of Action of Sinomenine and Its Derivatives: A Comprehensive Review" Molecules 29, no. 2: 540. https://doi.org/10.3390/molecules29020540
APA StyleHou, W., Huang, L., Huang, H., Liu, S., Dai, W., Tang, J., Chen, X., Lu, X., Zheng, Q., Zhou, Z., Zhang, Z., & Lan, J. (2024). Bioactivities and Mechanisms of Action of Sinomenine and Its Derivatives: A Comprehensive Review. Molecules, 29(2), 540. https://doi.org/10.3390/molecules29020540