Chemical Constituents and Bioactivities of the Plant-Derived Fungus Aspergillus fumigatus
Abstract
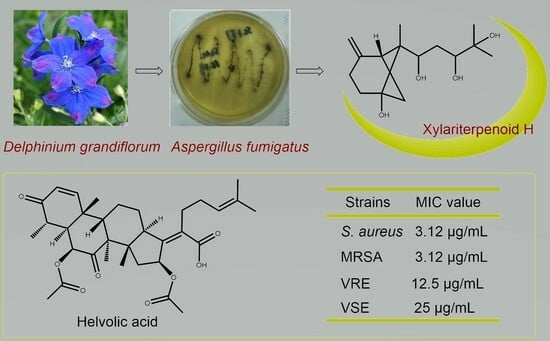
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization of Isolated Compounds
2.2. Antibacterial Activity
2.3. Cytotoxic Activity
2.4. Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
3.4. Cytotoxic Activity Assay
3.5. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.Z.; Chen, D.L.; Chen, Q.H.; Wang, F.P. Diterpenoid alkaloids from Delphinium majus. J. Nat. Prod. 2009, 72, 18–23. [Google Scholar] [CrossRef]
- Marin, C.; Ramirez-Macias, I.; Lopez-Cespedes, A.; Olmo, F.; Villegas, N.; Diaz, J.G.; Rosales, M.J.; Gutierrez-Sanchez, R.; Sanchez-Moreno, M. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against chagas disease. J. Nat. Prod. 2011, 74, 744–750. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, W.J.; Shi, Y.N.; Kennelly, E.J.; Zhao, D.K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat. Prod. Rep. 2020, 37, 763–796. [Google Scholar] [CrossRef]
- Li, T.X.; Meng, D.D.; Wang, Y.; An, J.L.; Bai, J.F.; Jia, X.W.; Xu, C.P. Antioxidant coumarin and pyrone derivatives from the insect-associated fungus Aspergillus versicolor. Nat. Prod. Res. 2020, 34, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Peng, X.P.; Li, G.; Wang, Q.; Lou, H.X. Naphtho-gamma-pyrones (NγPs) with obvious cholesterol absorption inhibitory activity from the marine-derived fungus Aspergillus niger S-48. Molecules 2022, 27, 2514. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.P.; Gong, M.; Li, Y.J.; Zhang, X.; Zhu, J.J.; Hu, C.H. 14-Membered resorcylic acid lactone derivatives with their anti-inflammatory from the fungus Aspergillus sp. ZJ-65. Fitoterapia 2021, 151, 104884. [Google Scholar] [CrossRef] [PubMed]
- Chaiyosang, B.; Kanokmedhakul, K.; Yodsing, N.; Boonlue, S.; Yang, J.X.; Wang, Y.A.; Andersen, R.J.; Yahuafai, J.; Kanokmedhakul, S. Three new indole diterpenoids from Aspergillus aculeatus KKU-CT2. Nat. Prod. Res. 2022, 36, 4973–4981. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, T.; Zhang, X.; Zhang, J.; Zhao, C. An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 2015, 20, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, W.; Wu, D.; He, W.; Zuo, M.; Wang, D.; Fu, P.; Wang, L.; Zhu, W. Sulfur-containing phenolic compounds from the cave soil-derived Aspergillus fumigatus GZWMJZ-152. J. Nat. Prod. 2022, 85, 433–440. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Chen, B.; Dai, H.; Sun, J.; Han, J.; Liu, H. Discovery of anti-MRSA secondary metabolites from a marine-derived fungus Aspergillus fumigatus. Mar. Drugs 2022, 20, 302. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.F.; Qin, L.L.; Li, X.H.; Cao, Z.X.; Gu, Y.C.; Guo, D.L.; Deng, Y. Two new sesquiterpenes produced by the endophytic fungus Aspergillus fumigatus from Ligusticum wallichii. J. Asian Nat. Prod. Res. 2020, 22, 138–143. [Google Scholar] [CrossRef] [PubMed]
- El-hawary, S.S.; Moawad, A.S.; Bahr, H.S.; Abdelmohsen, U.R.; Mohammed, R. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Adv. 2020, 10, 22058–22079. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, C.L.; Zhong, T.; Xu, W.; Luo, Z.H.; Shao, Z.; Yang, X.W. Sesquiterpenes from a deep-sea-derived fungus Graphostroma sp. MCCC 3A00421. Tetrahedron 2017, 73, 7267–7273. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Beih, A.A.; El-Halawany, A.M. Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae. Arch. Pharm. Res. 2012, 35, 1749–1756. [Google Scholar] [CrossRef]
- Han, J.; Liu, M.; Jenkins, I.D.; Liu, X.; Zhang, L.; Quinn, R.J.; Feng, Y. Genome-inspired chemical exploration of marine fungus Aspergillus fumigatus MF071. Mar. Drugs 2020, 18, 352. [Google Scholar] [CrossRef]
- Fujimoto, H.; Negishi, E.; Yamaguchi, K.; Nishi, N.; Yamazaki, M. Isolation of new tremorgenic metabolites from an ascomycete, Corynascus setosus. Chem. Pharm. Bull. 1996, 44, 1843–1848. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef]
- Yamazaki, M.; Fujimoto, H.; Kawasaki, T. Chemistry of tremorogenic metabolites. I. Fumitremorgin A from Aspergillus fumigatus. Chem. Pharm. Bull. 1980, 28, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Kalinovskii, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. Fumitremorgins from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2004, 40, 615–617. [Google Scholar] [CrossRef]
- Fill, T.P.; Rodrigues Asenha, H.B.; Marques, A.S.; Ferreira, A.G.; Rodrigues-Fo, E. Time course production of indole alkaloids by an endophytic strain of Penicillium brasilianum cultivated in rice. Nat. Prod. Res. 2013, 27, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.H.; Xu, S.; Liu, J.Y.; Ge, H.M.; Ding, H.; Xu, C.; Zhu, H.L.; Tan, R.X. Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006, 8, 5709–5712. [Google Scholar] [CrossRef]
- Lan, W.J.; Fu, S.J.; Xu, M.Y.; Liang, W.L.; Lam, C.K.; Zhong, G.H.; Xu, J.; Yang, D.P.; Li, H.J. Five new cytotoxic metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2016, 14, 18. [Google Scholar] [CrossRef]
- Odani, A.; Ishihara, K.; Ohtawa, M.; Tomoda, H.; Omura, S.; Nagamitsu, T. Total synthesis of pyripyropene A. Tetrahedron 2011, 67, 8195–8203. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Yang, J.; Li, H.; Zhou, F. Bioactive alkaloids of Aspergillus fumigatus, an endophytic fungus from Astragalus membranaceus. Chem. Nat. Compd. 2017, 53, 802–805. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovskii, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. Alkaloids from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2005, 41, 236–238. [Google Scholar] [CrossRef]
- Wang, M.; Huo, L.; Liu, H.; Zhao, L.; Xu, Z.; Tan, H.; Qiu, S.X. Thujasutchins N and O, two new compounds from the stems and roots of Thuja sutchuenensis. Nat. Prod. Res. 2022, 36, 2356–2362. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Goedecke, T.; Gunn, J.; Duan, J.A.; Che, C.T. Protostane and fusidane triterpenes: A mini-review. Molecules 2013, 18, 4054–4080. [Google Scholar] [CrossRef]
- Siala, W.; Rodriguez-Villalobos, H.; Fernandes, P.; Tulkens, P.M.; Van Bambeke, F. Activities of combinations of antistaphylococcal antibiotics with fusidic acid against staphylococcal biofilms in in vitro static and dynamic models. J. Antimicrob. Chemother. 2018, 62, e00598-18. [Google Scholar] [CrossRef]
- Long, J.; Ji, W.; Zhang, D.; Zhu, Y.; Bi, Y. Bioactivities and structure-activity relationships of fusidic acid derivatives: A review. Front. Pharmacol. 2021, 12, 759220. [Google Scholar] [CrossRef]
- Zhou, J.; Lancaster, L.; Donohue, J.P.; Noller, H.F. Crystal structures of EF-G-Ribosome complexes trapped in intermediate states of translocation. Science 2013, 340, 1236086. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.H.; Kalverda, A.P.; Calabrese, A.N. Fusidic acid resistance through changes in the dynamics of the drug target. Proc. Natl. Acad. Sci. USA 2020, 117, 25523–25531. [Google Scholar] [CrossRef]
- Lodeiro, S.; Xiong, Q.; Wilson, W.K.; Ivanova, Y.; Smith, M.L.; May, G.S.; Matsuda, S.P.T. Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus. Org. Lett. 2009, 11, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Mitsuguchi, H.; Seshime, Y.; Fujii, I.; Shibuya, M.; Ebizuka, Y.; Kushiro, T. Biosynthesis of steroidal antibiotic fusidanes: Functional analysis of oxidosqualene cyclase and subsequent tailoring enzymes from Aspergillus fumigatus. J. Am. Chem. Soc. 2009, 131, 6402–6411. [Google Scholar] [CrossRef]
- Lv, J.M.; Hu, D.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.D.; Wang, C.X.; Abe, I.; Yao, X.S. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat. Commun. 2017, 8, 1644. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, J.; Guimarães, R.; Palito, C.; Lemos, A.; Barros, L.; Alves, M.J. Alkaloids from Fungi. In Natural Secondary Metabolites: From Nature, Through Science, to Industry; Carocho, M., Heleno, S.A., Barros, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 529–554. [Google Scholar]
- Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from marine fungi: Promising antimicrobials. Antibiotics 2020, 9, 340. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.; Li, X.M.; Zhu, L.W.; Wang, B.G. Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. [Google Scholar] [CrossRef]
- Yan, L.-H.; Li, X.-M.; Chi, L.-P.; Li, X.; Wang, B.-G. Six new antimicrobial metabolites from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Mar. Drugs 2022, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, L.; Guo, M.; Jiang, Y.; Ding, Y.; Wang, Z.; Xin, X.; An, F. Bioactive indole diketopiperazine alkaloids from the marine endophytic fungus Aspergillus sp. YJ191021. Mar. Drugs 2021, 19, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.N.; Zhu, T.J.; Cai, S.X.; Gu, Q.Q.; Li, D.H. Three new indole-containing diketopiperazine alkaloids from a deep-ocean sediment derived fungus Penicillium griseofulvum. Helv. Chim. Acta 2010, 93, 1758–1763. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z.; et al. Raistrickindole A, an anti-HCV oxazinoindole alkaloid from Penicillium raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, K.; Ohashi, H.; Nishioka, K.; Yamasaki, M.; Furuta, M.; Mashiko, T.; Tomoshige, S.; Ohgane, K.; Kamisuki, S.; Watashi, K.; et al. Synthesis and antiviral activities of neoechinulin B and its derivatives. J. Nat. Prod. 2022, 85, 284–291. [Google Scholar] [CrossRef]
- Peng, J.; Lin, T.; Wang, W.; Xin, Z.; Zhu, T.; Gu, Q.; Li, D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013, 76, 1133–1140. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Burgio, G.; Thissera, B.; Orfali, R.; Jiffri, S.E.; Yaseen, M.; Sayed, A.M.; Rateb, M.E. Neoechinulin A as a promising SARS-CoV-2 Mpro inhibitor: In vitro and in silico study showing the ability of simulations in discerning active from inactive enzyme inhibitors. Mar. Drugs 2022, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Bahukhandi, A.; Dhyani, P.; Sati, P.; Capanoglu, E.; Docea, A.O.; Al-Harrasi, A.; Dey, A.; Calina, D. Therapeutic potential of neoechinulins and their derivatives: An overview of the molecular mechanisms behind pharmacological activities. Front. Nutr. 2021, 8, 664197. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Jiang, K.; Chen, B.; Chen, S.; Qi, X.; Lu, H.; Liu, J.; Zhou, X.; Gao, T.; Li, J.; et al. Evaluation of the anticarcinogenic potential of the endophyte, Streptomyces sp. LRE541 isolated from Lilium davidii var. unicolor (Hoog) Cotton. Microb. Cell Fact. 2021, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Ma, Y.; Chen, D.; Chen, P.; Hu, Y. Studies on structure and biological activity of indole diketopiperazine alkaloids. Prog. Chem. 2018, 30, 1067–1081. [Google Scholar]
- Fujimoto, H.; Sumino, M.; Okuyama, E.; Ishibashi, M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J. Nat. Prod. 2004, 67, 98–102. [Google Scholar] [CrossRef]
- Kuramochi, K.; Ohnishi, K.; Fujieda, S.; Nakajima, M.; Saitoh, Y.; Watanabe, N.; Takeuchi, T.; Nakazaki, A.; Sugawara, F.; Arai, T.; et al. Synthesis and biological activities of neoechinulin A derivatives: New aspects of structure-activity relationships for neoechinulin A. Chem. Pharm. Bull. 2008, 56, 1738–1743. [Google Scholar] [CrossRef]
- De Guzman, F.S.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New diketopiperazine metabolites from the sclerotia of Aspergillus ochraceus. J. Nat. Prod. 1992, 55, 931–939. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef]
- Boddapati, S.N.M.; Bollikolla, H.B.; Bhavani, K.G.; Saini, H.S.; Ramesh, N.; Jonnalagadda, S.B. Advances in synthesis and biological activities of quinazoline scaffold analogues: A review. Arab. J. Chem. 2023, 16, 105190. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. 3-Hydroxytyrosol regulates biofilm growth in Cunninghamella elegans. Fungal Biol. 2021, 125, 211–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hof, C.; Niemcova, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, H.; Chen, Y.; Guo, X.; Wang, W.; Guo, H.; Liu, Z.; Zhang, W. Cytorhizins A-D, four highly structure-combined benzophenones from the endophytic fungus Cytospora rhizophorae. Org. Lett. 2019, 21, 1063–1067. [Google Scholar] [CrossRef]



| Position | δC | δH (J in Hz) |
|---|---|---|
| 1 | 42.0, CH | 2.35, d (6.9) |
| 2 | 147.7, C | |
| 3 | 25.2, CH2 | 2.35, m 2.63, m |
| 4 | 31.7, CH2 | 1.79, m 1.98, m |
| 5 | 77.2, C | |
| 6 | 52.3, C | |
| 7 | 36.1, CH2 | 1.89, d (10.0) 2.51, dd (10.0, 6.9) |
| 8 | 70.4, CH | 4.66, m |
| 9 | 33.5, CH2 | 1.35, m 1.60, ddd (13.4, 10.4, 2.7) |
| 10 | 74.9, CH | 3.72, m |
| 11 | 73.2, C | |
| 12 | 23.9, CH3 | 1.20, s |
| 13 | 26.4, CH3 | 1.24, s |
| 14 | 10.5, CH3 | 0.82, s |
| 15 | 107.9, CH2 | 4.62, brs 4.68, brs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Z.; Zhang, Y.; Qiu, K.; Zheng, Y.; Chen, C.; Xu, L.; Lai, J.; Zou, Z.; Tan, H. Chemical Constituents and Bioactivities of the Plant-Derived Fungus Aspergillus fumigatus. Molecules 2024, 29, 649. https://doi.org/10.3390/molecules29030649
Sang Z, Zhang Y, Qiu K, Zheng Y, Chen C, Xu L, Lai J, Zou Z, Tan H. Chemical Constituents and Bioactivities of the Plant-Derived Fungus Aspergillus fumigatus. Molecules. 2024; 29(3):649. https://doi.org/10.3390/molecules29030649
Chicago/Turabian StyleSang, Zihuan, Yanjiang Zhang, Kaidi Qiu, Yuting Zheng, Chen Chen, Li Xu, Jiaying Lai, Zhenxing Zou, and Haibo Tan. 2024. "Chemical Constituents and Bioactivities of the Plant-Derived Fungus Aspergillus fumigatus" Molecules 29, no. 3: 649. https://doi.org/10.3390/molecules29030649
APA StyleSang, Z., Zhang, Y., Qiu, K., Zheng, Y., Chen, C., Xu, L., Lai, J., Zou, Z., & Tan, H. (2024). Chemical Constituents and Bioactivities of the Plant-Derived Fungus Aspergillus fumigatus. Molecules, 29(3), 649. https://doi.org/10.3390/molecules29030649