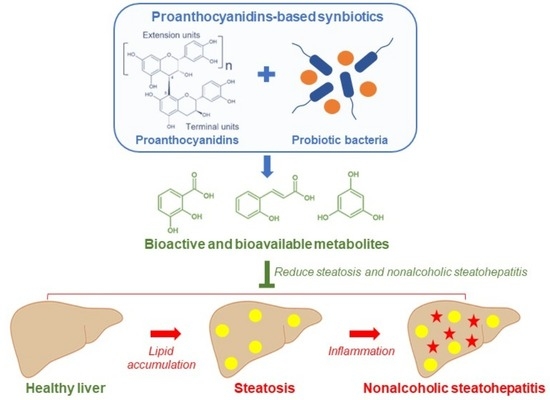
Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction
Abstract
:1. Introduction
2. Pathogenesis of Steatosis and NASH
2.1. Overexpression of FA Transporters
2.2. Disruption of FA Oxidation
2.3. TG Secretion in VLDL
2.4. Adipose Tissue Dysfunction
2.5. Steatosis Progression to NASH
2.5.1. ER Stress in NAFLD Progression
2.5.2. Oxidative Stress in NAFLD Progression
2.5.3. Adipose Tissue Dysfunction for NAFLD Progression
2.6. Gut Microbiota Dysbiosis in NAFLD Pathogenesis and Progression
3. Probiotic Microbe-Mediated Mechanisms for NAFLD Risk Reduction
4. PAC-Mediated Mechanisms for NAFLD Risk Reduction
5. PAC-Based Synbiotics for NAFLD Risk Reduction
6. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tariq, R.; Axley, P.; Singal, A.K. Extra-hepatic manifestations of nonalcoholic fatty liver disease: A review. J. Clin. Exp. Hepatol. 2020, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and prevention of hepatic steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and progression of non-alcoholic fatty liver disease: The role of advanced glycation end products. Int. J. Mol. Sci. 2019, 20, 5037. [Google Scholar] [CrossRef]
- Zuñiga-Aguilar, E.; Ramírez-Fernández, O. Fibrosis and hepatic regeneration mechanism. Transl. Gastroenterol. Hepatol. 2022, 7, 9. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular carcinoma: Special focus on fatty liver disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic fatty liver disease in adults: Current concepts in etiology, outcomes, and management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Ziółkowska, S.; Czarny, P.; Szemraj, J.; Pietras, T. The coexistence of nonalcoholic fatty liver disease and type 2 diabetes mellitus. J. Clin. Med. 2022, 11, 1375. [Google Scholar] [CrossRef]
- Rezayat, A.A.; Moghadam, M.D.; Nour, M.G.; Shirazinia, M.; Ghodsi, H.; Rouhbakhsh Zahmatkesh, M.R.; Tavakolizadeh Noghabi, M.; Hoseini, B.; Akhavan Rezayat, K. Association between smoking and non-alcoholic fatty liver disease: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312117745223. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, X.; Cao, X.; Miao, R.; Sun, Y.; Li, R.; Ye, J.; Zhong, B. Age patterns of nonalcoholic fatty liver disease incidence: Heterogeneous associations with metabolic changes. Diabetol. Metab. Syndr. 2022, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Torre, S.D. Non-alcoholic fatty liver disease as a canonical example of metabolic inflammatory-based liver disease showing a sex-specific prevalence: Relevance of estrogen signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. Race and ethnicity in non-alcoholic fatty liver disease (NAFLD): A narrative review. Nutrients 2022, 14, 4556. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Eapen, C.E. What are the current pharmacological therapies for nonalcoholic fatty liver disease? J. Clin. Exp. Hepatol. 2021, 11, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Nathani, R.R.; Bansal, M.B. Update on clinical trials for nonalcoholic steatohepatitis. Gastroenterol. Hepatol. 2023, 19, 371–381. [Google Scholar]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Rupasinghe, H.P.V. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015, 168, 595–605. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kohli, S.K.; Bali, S.; Sharma, M.; Kumar, R.; Bhardwaj, R.; Thukral, A.K. Differential distribution of polyphenols in plants using multivariate techniques. Biotechnol. Res. Innov. 2019, 3, 1–21. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V. Special issue “flavonoids and their disease prevention and treatment potential”: Recent advances and future perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. BioFactors 2016, 42, 5–12. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Vazquez-Flores, A.A.; Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; González-Aguilar, G.A.; Aguilar, C.N. Proanthocyanidins with a low degree of polymerization are good inhibitors of digestive enzymes because of their ability to form specific interactions: A hypothesis. J. Food Sci. 2018, 83, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Stürzenbaum, S.R. Proanthocyanidins of natural origin: Molecular mechanisms and implications for lipid disorder and aging-associated diseases. Adv. Nutr. 2019, 10, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef]
- Ou, K.; Sarnoski, P.; Schneider, K.R.; Song, K.; Khoo, C.; Gu, L. Microbial catabolism of procyanidins by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Kohzaki, H.; Shirato, M.; Shishido, S.; Nakamura, K. Metabolic fate of orally ingested proanthocyanidins through the digestive tract. Antioxidants 2022, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.P.D.W.; Langille, M.G.I.; Rupasinghe, H.P.V. Hepatotoxicity of polymeric proanthocyanidins is caused by translocation of bacterial lipopolysaccharides through impaired gut epithelium. Toxicol. Lett. 2023, 379, 35–47. [Google Scholar] [CrossRef]
- Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V. Microbial metabolites of proanthocyanidins reduce chemical carcinogen-induced dna damage in human lung epithelial and fetal hepatic cells in vitro. Food Chem. Toxicol. 2019, 125, 479–493. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, T.; Hung, Y.-H.; Carreiro, A.; Buhman, K.K. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, B.; Hesselink, M.K.C.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 2016, 59, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Herrmann, T.; Seeßle, J.; Liebisch, G.; Merle, U.; Stremmel, W.; Chamulitrat, W. Role of fatty acid transport protein 4 in metabolic tissues: Insights into obesity and fatty liver disease. Biosci. Rep. 2022, 42, BSR20211854. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.-S.; Wu, Y.-Z.; Gan, Y.-W.; Wang, H.-L.; Wu, L.-J.; Zheng, C.-M.; Ming, Y.; Xiong, R.; Li, Y.-L.; Lei, S.-H.; et al. Andrographolide ameliorates hepatic steatosis by suppressing FATP2-mediated fatty acid uptake in mice with nonalcoholic fatty liver disease. J. Nat. Med. 2023, 77, 73–86. [Google Scholar] [CrossRef]
- Miquilena-Colina, M.E.; Lima-Cabello, E.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Fernández-Bermejo, M.; Lozano-Rodríguez, T.; Vargas-Castrillón, J.; Buqué, X.; Ochoa, B.; Aspichueta, P.; et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011, 60, 1394–1402. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, H.; Liao, M.; Zheng, E.; Luo, X.; Xiao, A.; Li, Y.; Chen, L.; Wei, L.; Zhao, L.; et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol. Metab. 2022, 57, 101428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, C.; Luo, X.; Wang, P.; Zhou, W.; Zhong, S.; Xie, Y.; Jiang, Y.; Yang, P.; Tang, R.; et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 2018, 69, 705–717. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid β-oxidation by increasing its localization to mitochondria and interaction with long-chain acyl-coa synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Gieseler, R.K.; Sowa, J.-P.; Kahraman, A.; Erhard, J.; Wedemeyer, I.; Emons, B.; Jochum, C.; Feldkamp, T.; Gerken, G.; et al. Apoptosis is associated with CD36/Fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010, 30, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a Signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Meshulam, T.; Simard, J.R.; Wharton, J.; Hamilton, J.A.; Pilch, P.F. Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry 2006, 45, 2882–2893. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Chen, H.-T.; Yu, C.-H.; Teng, X.-D.; Yao, H.-T.; Xu, G.-Q. Upregulation of caveolin-1 and sr-b1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 630–636. [Google Scholar] [CrossRef]
- Ring, A.; Le Lay, S.; Pohl, J.; Verkade, P.; Stremmel, W. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim. Biophys. Acta 2006, 1761, 416–423. [Google Scholar] [CrossRef]
- Takeda, M.; Sakaguchi, T.; Hiraide, T.; Shibasaki, Y.; Morita, Y.; Kikuchi, H.; Ikegami, K.; Setou, M.; Konno, H.; Takeuchi, H. Role of caveolin-1 in hepatocellular carcinoma arising from non-alcoholic fatty liver disease. Cancer Sci. 2018, 109, 2401–2411. [Google Scholar] [CrossRef]
- Xue, W.; Wang, J.; Jiang, W.; Shi, C.; Wang, X.; Huang, Y.; Hu, C. Caveolin-1 alleviates lipid accumulation in nafld associated with promoting autophagy by inhibiting the Akt/mTOR pathway. Eur. J. Pharmacol. 2020, 871, 172910. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The importance of the fatty acid transporter l-carnitine in non-alcoholic fatty liver disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Naguib, G.; Morris, N.; Yang, S.; Fryzek, N.; Haynes-Williams, V.; Huang, W.C.A.; Norman-Wheeler, J.; Rotman, Y. Dietary fatty acid oxidation is decreased in non-alcoholic fatty liver disease: A palmitate breath test study. Liver Int. 2020, 40, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol. Metab. Syndr. 2011, 3, 31. [Google Scholar] [CrossRef]
- Tokoro, M.; Gotoh, K.; Kudo, Y.; Hirashita, Y.; Iwao, M.; Arakawa, M.; Endo, M.; Oribe, J.; Masaki, T.; Honda, K.; et al. α-Tocopherol suppresses hepatic steatosis by increasing CPT-1 expression in a mouse model of diet-induced nonalcoholic fatty liver disease. Obes. Sci. Pract. 2020, 7, 91–99. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.-R.; Van Hul, W.; Mertens, I.; et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Gomaraschi, M.; Fracanzani, A.L.; Dongiovanni, P.; Pavanello, C.; Giorgio, E.; Da Dalt, L.; Norata, G.D.; Calabresi, L.; Consonni, D.; Lombardi, R.; et al. Lipid accumulation impairs lysosomal acid lipase activity in hepatocytes: Evidence in nafld patients and cell cultures. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 158523. [Google Scholar] [CrossRef]
- Fujita, K.; Nozaki, Y.; Wada, K.; Yoneda, M.; Fujimoto, Y.; Fujitake, M.; Endo, H.; Takahashi, H.; Inamori, M.; Kobayashi, N.; et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 2009, 50, 772–780. [Google Scholar] [CrossRef]
- Shelness, G.S.; Sellers, J.A. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001, 12, 151. [Google Scholar] [CrossRef]
- Tanoli, T.; Yue, P.; Yablonskiy, D.; Schonfeld, G. Fatty liver in familial hypobetalipoproteinemia: Roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J. Lipid Res. 2004, 45, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Berriot-Varoqueaux, N.; Aggerbeck, L.P.; Samson-Bouma, M.; Wetterau, J.R. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000, 20, 663–697. [Google Scholar] [CrossRef] [PubMed]
- Lytle, K.A.; Bush, N.C.; Triay, J.M.; Kellogg, T.A.; Kendrick, M.L.; Swain, J.M.; Gathaiya, N.W.; Hames, K.C.; Jensen, M.D. Hepatic fatty acid balance and hepatic fat content in humans with severe obesity. J. Clin. Endocrinol. Metab. 2019, 104, 6171–6181. [Google Scholar] [CrossRef] [PubMed]
- Richard, P. The role of adipose tissue in fatty liver diseases. Liver Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Mahmoud, O.M.; Mahmoud, G.A.E.; Atta, H.; Abbas, W.A.; Ahmed, H.M.; Abozaid, M.A.A. Visceral and subcutaneous fat, muscle mass, and liver volume as noninvasive predictors of the progress of non-alcoholic fatty liver disease. Egypt. J. Radiol. Nucl. Med. 2023, 54, 5. [Google Scholar] [CrossRef]
- Igarashi, Y.; Tanaka, M.; Okada, H.; Hashimoto, Y.; Kumagai, M.; Yamaoka, M.; Nishimura, H.; Fukui, M. Visceral adipose tissue quality was associated with nonalcoholic fatty liver disease, independent of its quantity. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Suchacki, K.J.; Stimson, R.H. Nutritional regulation of human brown adipose tissue. Nutrients 2021, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Tingelstad, H.C.; Noll, C.; Frisch, F.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Richard, D.; Haman, F.; Carpentier, A.C. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat. Commun. 2017, 8, 14146. [Google Scholar] [CrossRef] [PubMed]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Falkevall, A.; Mehlem, A.; Folestad, E.; Ning, F.C.; Osorio-Conles, Ó.; Radmann, R.; de Hollanda, A.; Wright, S.D.; Scotney, P.; Nash, A.; et al. Inhibition of VEGF-B signaling prevents non-alcoholic fatty liver disease development by targeting lipolysis in the white adipose tissue. J. Hepatol. 2023, 78, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Skat-Rørdam, J.; Højland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Morán-Salvador, E.; López-Parra, M.; García-Alonso, V.; Titos, E.; Martínez-Clemente, M.; González-Périz, A.; López-Vicario, C.; Barak, Y.; Arroyo, V.; Clària, J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011, 25, 2538–2550. [Google Scholar] [CrossRef]
- Giorgio, V.; Prono, F.; Graziano, F.; Nobili, V. Pediatric non alcoholic fatty liver disease: Old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013, 13, 40. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Rennert, C.; Heil, T.; Schicht, G.; Stilkerich, A.; Seidemann, L.; Kegel-Hübner, V.; Seehofer, D.; Damm, G. Prolonged lipid accumulation in cultured primary human hepatocytes rather leads to ER stress than oxidative stress. Int. J. Mol. Sci. 2020, 21, 7097. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Shinjo, S.; Jiang, S.; Nameta, M.; Suzuki, T.; Kanai, M.; Nomura, Y.; Goda, N. Disruption of the mitochondria-associated ER membrane (MAM) plays a central role in palmitic acid–induced insulin resistance. Exp. Cell Res. 2017, 359, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Tsai, P.-J.; Chen, P.-H.; Ye, M.; Guo, J.; Su, Z. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Malhi, H. The unfolded protein response and hepatic lipid metabolism in non-alcoholic fatty liver disease. Pharmacol. Ther. 2019, 203, 107401. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Chen, P.; Li, Y.; Liu, S.; Liu, Q.; Zhang, H.; Wu, Z.; Song, K.; Liu, J.; et al. The ER stress sensor inositol-requiring enzyme 1α in kupffer cells promotes hepatic ischemia-reperfusion injury. J. Biol. Chem. 2022, 298, 101532. [Google Scholar] [CrossRef]
- DeZwaan-McCabe, D.; Sheldon, R.D.; Gorecki, M.C.; Guo, D.-F.; Gansemer, E.R.; Kaufman, R.J.; Rahmouni, K.; Gillum, M.P.; Taylor, E.B.; Teesch, L.M.; et al. ER stress inhibits liver fatty acid oxidation while unmitigated stress leads to anorexia-induced lipolysis and both liver and kidney steatosis. Cell Rep. 2017, 19, 1794–1806. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, D.; Kwak, J.-H.; Lee, S.; Lee, Y.-H.; Yun, J.; Son, T.G.; Jung, Y.-S. Tunicamycin-induced er stress is accompanied with oxidative stress via abrogation of sulfur amino acids metabolism in the liver. Int. J. Mol. Sci. 2018, 19, 4114. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Podszun, M.C.; Alawad, A.S.; Lingala, S.; Morris, N.; Huang, W.-C.A.; Yang, S.; Schoenfeld, M.; Rolt, A.; Ouwerkerk, R.; Valdez, K.; et al. Vitamin E treatment in nafld patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020, 37, 101710. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Malhi, H.; Guicciardi, M.E.; Gores, G.J. Hepatocyte death: A clear and present danger. Physiol. Rev. 2010, 90, 1165–1194. [Google Scholar] [CrossRef]
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxidative Med. Cell. Longev. 2016, 2016, e4234061. [Google Scholar] [CrossRef]
- Nan, B.; Yang, C.; Li, L.; Ye, H.; Yan, H.; Wang, M.; Yuan, Y. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem. Toxicol. 2021, 148, 111937. [Google Scholar] [CrossRef] [PubMed]
- Panahi, G.; Pasalar, P.; Zare, M.; Rizzuto, R.; Meshkani, R. High glucose induces inflammatory responses in HepG2 cells via the oxidative stress-mediated activation of NF-κB, and MAPK pathways in HepG2 cells. Arch. Physiol. Biochem. 2018, 124, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Huang, H.; Xie, H.; Huang, M.; Jiang, W.; Ding, B.; Zhu, Q. TNF-α/TNFR1 regulates the polarization of Kupffer cells to mediate trichloroethylene-induced liver injury. Ecotoxicol. Environ. Saf. 2022, 230, 113141. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-Y.; Kim, D.-H.; Chun, K.-H. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Lab. Anim. Res. 2018, 34, 133–139. [Google Scholar] [CrossRef]
- Maeda, H.; Ishima, Y.; Saruwatari, J.; Mizuta, Y.; Minayoshi, Y.; Ichimizu, S.; Yanagisawa, H.; Nagasaki, T.; Yasuda, K.; Oshiro, S.; et al. Nitric oxide facilitates the targeting Kupffer cells of a nano-antioxidant for the treatment of NASH. J. Control. Release 2022, 341, 457–474. [Google Scholar] [CrossRef]
- Gandhi, C.R. Oxidative stress and hepatic stellate cells: A paradoxical relationship. Trends Cell Mol. Biol. 2012, 7, 1–10. [Google Scholar]
- Alkhouri, N.; Gornicka, A.; Berk, M.P.; Thapaliya, S.; Dixon, L.J.; Kashyap, S.; Schauer, P.R.; Feldstein, A.E. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J. Biol. Chem. 2010, 285, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Samovski, D.; Smith, G.I.; Cifarelli, V.; Farabi, S.S.; Yoshino, J.; Pietka, T.; Chang, S.-W.; Ghosh, S.; Myckatyn, T.M.; et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 2021, 161, 968–981.e12. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Perakakis, N.; Mantzoros, C.S. Association of adipokines with development and progression of nonalcoholic fatty liver disease. Endocrinol. Metab. 2018, 33, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Toulis, K.A.; Goulis, D.G.; Zavos, C.; Kountouras, J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2011, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Zavos, C. The multi-hit process and the antagonistic roles of tumor necrosis factor-alpha and adiponectin in non alcoholic fatty liver disease. Hippokratia 2009, 13, 127. [Google Scholar] [PubMed]
- Rotundo, L.; Persaud, A.; Feurdean, M.; Ahlawat, S.; Kim, H.-S. The association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin. Mol. Hepatol. 2018, 24, 392–401. [Google Scholar] [CrossRef]
- Petrescu, A.D.; Grant, S.; Williams, E.; An, S.Y.; Seth, N.; Shell, M.; Amundsen, T.; Tan, C.; Nadeem, Y.; Tjahja, M.; et al. Leptin enhances hepatic fibrosis and inflammation in a mouse model of cholestasis. Am. J. Pathol. 2022, 192, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V.; Ridgway, N.D. Mechanisms by which probiotic bacteria attenuate the risk of hepatocellular carcinoma. Int. J. Mol. Sci. 2021, 22, 2606. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut microbiome and its impact on obesity and obesity-related disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Shin, M.J.; Youn, G.S.; Yoon, S.J.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Han, S.H.; Kim, B.K.; Lee, D.Y.; et al. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin. Mol. Hepatol. 2021, 27, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Yu, J.S.; Min, B.H.; Gupta, H.; Won, S.-M.; Park, H.J.; Han, S.H.; Kim, B.-Y.; Kim, K.H.; Kim, B.K.; et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front. Microbiol. 2023, 14, 1129904. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.-J.; Wang, X.-Y.; Fan, J.-G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Vasques-Monteiro, I.M.L.; Silva-Veiga, F.M.; Miranda, C.S.; de Andrade Gonçalves, É.C.B.; Daleprane, J.B.; Souza-Mello, V. A rise in proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 2021, 91, 26–35. [Google Scholar] [CrossRef]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Scott Rector, R.; Staveley-O’Carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef]
- Chen, J.; Thomsen, M.; Vitetta, L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J. Cell. Biochem. 2019, 120, 2713–2720. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef]
- Schmitt, J.; Kong, B.; Stieger, B.; Tschopp, O.; Schultze, S.M.; Rau, M.; Weber, A.; Müllhaupt, B.; Guo, G.L.; Geier, A. Protective effects of farnesoid X receptor (FXR) on Hepatic Lipid Accumulation Are Mediated by Hepatic FXR and independent of intestinal FGF15 signal. Liver Int. 2015, 35, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Torra, I.P.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.-C.; Staels, B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against nafld via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Zampella, A.; Ricci, P.; Distrutti, E.; Biagioli, M. Immunomodulatory functions of FXR. Mol. Cell. Endocrinol. 2022, 551, 111650. [Google Scholar] [CrossRef]
- Guo, C.; Chen, W.-D.; Wang, Y.-D. TGR5, not only a metabolic regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Chen, W.-D.; Yu, D.; Forman, B.M.; Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef]
- Xie, G.; Jiang, R.; Wang, X.; Liu, P.; Zhao, A.; Wu, Y.; Huang, F.; Liu, Z.; Rajani, C.; Zheng, X.; et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine 2021, 66, 103290. [Google Scholar] [CrossRef]
- Aoki, R.; Onuki, M.; Hattori, K.; Ito, M.; Yamada, T.; Kamikado, K.; Kim, Y.-G.; Nakamoto, N.; Kimura, I.; Clarke, J.M.; et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome 2021, 9, 188. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, X.; Zhao, Z.; Liao, Y.; Zhou, T.; Xiang, Q. A Potential link between plasma short-chain fatty acids, TNF-α level and disease progression in non-alcoholic fatty liver disease: A retrospective study. Exp. Ther. Med. 2022, 24, 598. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human nafld as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New insights into gut-bacteria-derived indole and its derivatives in intestinal and liver diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Neyrinck, A.M.; Olivares, M.; Rodriguez, J.; de Rocca Serra, A.; Roumain, M.; Bindels, L.B.; Cani, P.D.; Evenepoel, P.; Muccioli, G.G.; et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018, 32, 6681–6693. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and nash. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Li, H.; Zhao, J.; Wei, X.; Lin, W.; Zhao, X.; Jiang, A.; Yuan, J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020, 35, 2009–2019. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, S.-E.; Lee, N.Y.; Kim, J.-H.; Jung, J.-H.; Jang, M.-K.; Park, S.-H.; Lee, M.-S.; Kim, D.-J.; Kim, H.-S.; et al. Role of microbiota-derived metabolites in alcoholic and non-alcoholic fatty liver diseases. Int. J. Mol. Sci. 2022, 23, 426. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio 2020, 11, e03263-19. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Mckenzie, T.J.; Lillegard, J.B.; Yu, Y.; Juskewitch, J.E.; Nedredal, G.I.; Brunn, G.J.; Yi, E.S.; Smyrk, T.C.; Nyberg, S.L. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J. Surg. Res. 2013, 180, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, C.R. Pro- and anti-fibrogenic functions of gram-negative bacterial lipopolysaccharide in the liver. Front. Med. 2020, 7, 130. [Google Scholar] [CrossRef]
- Fukunishi, S.; Sujishi, T.; Takeshita, A.; Ohama, H.; Tsuchimoto, Y.; Asai, A.; Tsuda, Y.; Higuchi, K. Lipopolysaccharides accelerate hepatic steatosis in the development of nonalcoholic fatty liver disease in Zucker rats. J. Clin. Biochem. Nutr. 2014, 54, 39–44. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Yang, W.; Ling, W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy 2017, 13, 1813–1827. [Google Scholar] [CrossRef]
- Cho, Y.-E.; Kim, D.-K.; Seo, W.; Gao, B.; Yoo, S.-H.; Song, B.-J. Fructose promotes leaky gut, endotoxemia and liver fibrosis through CYP2E1-mediated oxidative and nitrative stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef]
- Milner, E.; Stevens, B.; An, M.; Lam, V.; Ainsworth, M.; Dihle, P.; Stearns, J.; Dombrowski, A.; Rego, D.; Segars, K. Utilizing probiotics for the prevention and treatment of gastrointestinal diseases. Front. Microbiol. 2021, 12, 689958. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Gu, Y.; Li, X.; Chen, H.; Sun, Y.; Yang, L.; Ma, Y.; Yong Chan, E.C. Antidiabetic effects of multi-species probiotic and its fermented milk in mice via restoring gut microbiota and intestinal barrier. Food Biosci. 2022, 47, 101619. [Google Scholar] [CrossRef]
- Chen, B.; Sun, L.; Zeng, G.; Shen, Z.; Wang, K.; Yin, L.; Xu, F.; Wang, P.; Ding, Y.; Nie, Q.; et al. Gut bacteria alleviate smoking-related nash by degrading gut nicotine. Nature 2022, 610, 562–568. [Google Scholar] [CrossRef]
- Ahn, S.B.; Jun, D.W.; Kang, B.-K.; Lim, J.H.; Lim, S.; Chung, M.-J. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef]
- Cai, G.; Su, H.; Zhang, J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine 2020, 99, e21464. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, W.; Shan, M.; Lu, Z.; Lu, Y. Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice. Food Sci. Hum. Wellness 2022, 11, 1428–1439. [Google Scholar] [CrossRef]
- Hashemnia, S.M.R.; Meshkani, R.; Zamani-Garmsiri, F.; Shabani, M.; Tajabadi-Ebrahimi, M.; Ragerdi Kashani, I.; Siadat, S.D.; Mohassel Azadi, S.; Emamgholipour, S. Amelioration of obesity-induced white adipose tissue inflammation by Bacillus coagulans T4 in a high-fat diet-induced obese murine model. Life Sci. 2023, 314, 121286. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Tang, C.; Meng, F.; Pang, X.; Chen, M.; Zhou, L.; Lu, Z.; Lu, Y. Protective effects of lactobacillus acidophilus NX2-6 against oleic acid-induced steatosis, mitochondrial dysfunction, endoplasmic reticulum stress and inflammatory responses. J. Funct. Foods 2020, 74, 104206. [Google Scholar] [CrossRef]
- Arai, N.; Miura, K.; Aizawa, K.; Sekiya, M.; Nagayama, M.; Sakamoto, H.; Maeda, H.; Morimoto, N.; Iwamoto, S.; Yamamoto, H. Probiotics suppress nonalcoholic steatohepatitis and carcinogenesis progression in hepatocyte-specific PTEN knockout mice. Sci. Rep. 2022, 12, 16206. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yan, J.; Wu, L.; Wu, J.; Chen, Z.; Jiang, J.; Chen, Z.; He, B. Probiotics alleviated nonalcoholic fatty liver disease in high-fat diet-fed rats via gut microbiota/FXR/FGF15 signaling pathway. J. Immunol. Res. 2021, 2021, 2264737. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.J.; Monetti, M.; Shih, M.; Zhou, P.; Watkins, S.M.; Bhanot, S.; Farese, R.V. A specific role for dgat1 in hepatic steatosis due to exogenous fatty acids. Hepatology 2009, 50, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rao, H.; Liu, F.; Wei, L.; Li, H.; Wu, C. Recent advances in adipose tissue dysfunction and its role in the pathogenesis of non-alcoholic fatty liver disease. Cells 2021, 10, 3300. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, A.; Kataoka, E.; Sasaki, T.; Hamada, K.; Sasaki, J.; Mizuno, K.; Hasegawa, G.; Kishimoto, H.; Iizuka, M.; et al. Hepatocyte-specific pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Investig. 2004, 113, 1774–1783. [Google Scholar] [CrossRef]
- Thiele, N.D.; Wirth, J.W.; Steins, D.; Koop, A.C.; Ittrich, H.; Lohse, A.W.; Kluwe, J. TIMP-1 is upregulated, but not essential in hepatic fibrogenesis and carcinogenesis in mice. Sci. Rep. 2017, 7, 714. [Google Scholar] [CrossRef]
- Rockey, D.C.; Du, Q.; Shi, Z. Smooth muscle α-actin deficiency leads to decreased liver fibrosis via impaired cytoskeletal signaling in hepatic stellate cells. Am. J. Pathol. 2019, 189, 2209–2220. [Google Scholar] [CrossRef]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: Recent updates. Int. J. Biol. Sci. 2021, 17, 818–833. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics Improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mojiri-Forushani, H.; Hemmati, A.; Khanzadeh, A.; Zahedi, A. Effectiveness of grape seed extract in patients with nonalcoholic fatty liver: A randomized double-blind clinical study. Hepat. Mon. 2022, 22, e132309. [Google Scholar] [CrossRef]
- Wang, M.; Mao, H.; Chen, J.; Li, Q.; Ma, W.; Zhu, N.; Qi, L.; Wang, J. Chinese Bayberry (Myrica rubra sieb. et Zucc.) leaves proanthocyanidins alleviate insulin-resistance via activating PI3K/AKT pathway in HepG2 cells. J. Funct. Foods 2022, 99, 105297. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Chen, J.; Ye, X. Proanthocyanidins from chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves regulate lipid metabolism and glucose consumption by activating AMPK Pathway in HepG2 cells. J. Funct. Foods 2017, 29, 217–225. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; El-Jalbout, R.; Sauvé, M.F.; Ahmarani, L.; Sané, A.T.; Ould-Chikh, N.-E.-H.; N’Timbane, T.; Patey, N.; Desjardins, Y.; et al. Cranberry proanthocyanidins as a therapeutic strategy to curb metabolic syndrome and fatty liver-associated disorders. Antioxidants 2023, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Gu, A.X.; Huang, B.Y.; Zhang, T.; Li, J.P.; Shan, A.S. Dietary grape seed proanthocyanidin alleviates the liver injury induced by long-term high-fat diets in Sprague Dawley rats. Front. Vet. Sci. 2022, 9, 959906. [Google Scholar] [CrossRef] [PubMed]
- Yogalakshmi, B.; Sreeja, S.; Geetha, R.; Radika, M.K.; Anuradha, C.V. Grape seed proanthocyanidin rescues rats from steatosis: A comparative and combination study with metformin. J. Lipids 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mo, R.; Zhang, M.; Zhou, W.; Li, D. Grape seed proanthocyanidin alleviates intestinal inflammation through gut microbiota-bile acid crosstalk in mice. Front. Nutr. 2022, 8, 786682. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, C.; Xu, H.; Wu, Q.; Zhou, Y.; Zhou, X.; Miao, J. Procyanidin B2 prevents dyslipidemia via modulation of gut microbiome and related metabolites in high-fat diet fed mice. J. Funct. Foods 2020, 75, 104285. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Ye, X.; Chen, S. Proanthocyanidins from chinese bayberry leaves reduce obesity and associated metabolic disorders in high-fat diet-induced obese mice through a combination of AMPK activation and an alteration in gut microbiota. Food Funct. 2022, 13, 2295–2305. [Google Scholar] [CrossRef]
- Amer, M.A.; Othman, A.I.; EL-Missiry, M.A.; Farag, A.A.; Amer, M.E. Proanthocyanidins attenuated liver damage and suppressed fibrosis in CCl4-treated rats. Environ. Sci. Pollut. Res. 2022, 29, 91127–91138. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Y.-L.; Li, X.; Zhang, Y.; Xia, K.-L.; Cui, B.-W.; Lian, L.-H.; Nan, J.-X. Oligomeric Proanthocyanidin derived from grape seeds inhibited NF-κB signaling in activated HSC: Involvement of JNK/ERK MAPK and PI3K/Akt pathways. Biomed. Pharmacother. 2017, 93, 674–680. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study1,2,3. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V. Proanthocyanidins biotransformed by Saccharomyces cerevisiae prevent the pathogenesis of steatosis and progression to steatohepatitis in vitro. J. Funct. Foods 2024, 112, 105961. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Lee, H.G.; Seo, K.-H.; Yokoyama, W.; Kim, H. Antiobesity effect of prebiotic polyphenol-rich grape seed flour supplemented with probiotic kefir-derived lactic acid bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-H.; Lee, H.G.; Seo, K.-H.; Kim, H. Combination of whole grapeseed flour and newly isolated kefir lactic acid bacteria reduces high-fat-induced hepatic steatosis. Mol. Nutr. Food Res. 2019, 63, 1801040. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-H.; Kim, D.-H.; Yokoyama, W.H.; Kim, H. Synbiotic Effect of Whole Grape Seed Flour and Newly Isolated Kefir Lactic Acid Bacteria on Intestinal Microbiota of Diet-Induced Obese Mice. J. Agric. Food Chem. 2020, 68, 13131–13137. [Google Scholar] [CrossRef]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green tea powder and lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, M.; Kumari, A.; Sharma, A.; Gulati, A.; Gupta, M.; Padwad, Y. Diet supplemented with phytochemical epigallocatechin gallate and probiotic lactobacillus fermentum confers second generation synbiotic effects by modulating cellular immune responses and antioxidant capacity in aging mice. Eur. J. Nutr. 2019, 58, 2943–2957. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 pathway in liver diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the mechanism of the health benefits of proanthocyanidins: Absorption, metabolism, and interaction with gut microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Li, W.; You, B.; Yu, J.; Huang, X.; Yang, R. Gut microbiota composition affects procyanidin A2-attenuated atherosclerosis in ApoE–/– mice by modulating the bioavailability of its microbial metabolites. J. Agric. Food Chem. 2021, 69, 6989–6999. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Parmar, I.; Neir, S.V. Biotransformation of cranberry proanthocyanidins to probiotic metabolites by Lactobacillus rhamnosus enhances their anticancer activity in HepG2 cells in vitro. Oxidative Med. Cell Longev. 2019, 2019, 4750795. [Google Scholar] [CrossRef] [PubMed]
- Özcan, E.; Rozycki, M.R.; Sela, D.A. Cranberry proanthocyanidins and dietary oligosaccharides synergistically modulate Lactobacillus plantarum physiology. Microorganisms 2021, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The effects of different degrees of procyanidin polymerization on the nutrient absorption and digestive enzyme activity in mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Condensed tannins decreased the growth performance and impaired intestinal immune function in on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2020, 123, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, K.R.; Downs, C.T.; Nsahlai, I.V. The effects of graded levels of dietary tannin on the epithelial tissue of the gastro-intestinal tract and liver and kidney masses of boer goats. Anim. Sci. 2002, 74, 579–586. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The many faces of Enterococcus spp.—Commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Amadoro, C.; Colavita, G. Members of the Lactobacillus genus complex (LGC) as opportunistic pathogens: A review. Microorganisms 2019, 7, 126. [Google Scholar] [CrossRef]



| Probiotics | Experimental Model | Biological Functions and Mechanisms | Reference |
|---|---|---|---|
| (1) A mixture of L. acidophilus, L. rhamnosus, L. paracasei, P. pentosaceus, B. lactis, and B. breve. | Obese NAFLD patients were supplemented with the probiotic bacteria mixture (1 × 109 cfu/day) for 12 weeks. |
| [154] |
| (2) A mixture of Lactobacillus, Bifidobacterium, and Enterococcus probiotic bacteria. | NAFLD patients were supplemented with the probiotic bacteria (1 g twice per day) concomitantly with a low-calorie diet and exercise therapy for three months. |
| [155] |
| (3) L. plantarum Q16. | Mice induced for NAFLD by feeding a high-fat diet were supplemented with the probiotic bacteria (1 × 109 cfu/day) for 8 weeks. |
| [156] |
| (4) Bacillus coagulans T4. | C57BL/6J mice were fed a high-fat diet for 10 weeks to induce obesity. Then, the mice were supplemented with the probiotic bacteria (1 × 109 cfu/day) for 8 weeks while feeding with a high-fat diet. |
| [157] |
| |||
| (5) L. casei, L. fermentum, L. acidophilus, L. rhamnosus, and L. paracesei. | C57BL/6J mice induced for obesity by feeding with a high-fat diet were administered with the different strains of probiotic bacteria separately. |
| [158] |
| (6) L. acidophilus cell-free extract. | HepG2 cells were treated with the cell-free extract of probiotic bacteria for 24 h. Then, the cells were exposed to oleic acid (0.9 mM) for 24 h to induce lipid accumulation. |
| [159] |
| (7) A mixture of 20 strains of lactic acid bacteria. | C57BL/6 mice of hepatocyte-specific phosphate and tensin homolog (PTEN) gene knockout were supplemented. |
| [160] |
| (8) L. acidophilus. | C57BL/6J mice were fed a high-fat diet for 11 weeks. Then, supplemented with the probiotic bacteria (5 × 109 cfu/day) and the high-fat diet concomitantly for another week. |
| [161] |
| (9) L. acidophilus tablet. | Sprague Dawley rats were fed a high-fat diet for 6-weeks. Then, the rats were supplemented with the probiotic tablet (312 mg/kg of body weight/day, 1 × 107 cfu/g of tablet) and high-fat diet concomitantly for 8 weeks. |
| [162] |
| Experimental Model | Biological Functions and Mechanisms | Reference |
|---|---|---|
| (1) Supplementation of NAFLD patients with PAC-rich grape seed extract (200 mg twice per day) for 2 months. |
| [171] |
| (2) C57BL/6 mice were supplemented with the cranberry PAC (200 mg/kg bw/day) and an HFHS diet concomitantly for 12 weeks. |
| [174] |
| (3) Wistar rats induced for NAFLD by feeding with an HFHF diet for 30 days were supplemented with grape seed PAC (100 mg/kg bw/day) for another 15 days concomitantly with the HFHF diet. |
| [176] |
| (4) Sprague Dawley rats were fed with an HFD for 8 weeks to induce obesity. The mice were supplemented with grape seed PAC (500 mg/kg bw/day) and HFD concomitantly for 4 weeks. |
| [175] |
| (5) HepG2 and L02 liver cells were exposed to a mixture of oleic and palmitic acids (0.2 mM for 24 h) and treated with procyanidin B2 (2.5–10 µg/mL for 24 h). C57BL/6 mice were fed with an HFD for 10 weeks to induce obesity. Then, mice were administered procyanidin B2 (50 and 150 mg/kg bw/day) and fed with the HFD concomitantly for 10 weeks. |
| [177] |
| (6) An LPS-injected mouse model to evaluate intestinal inflammation. C57BL/6 mice were supplemented with grape seed PAC (250 mg/kg bw/day) for 20 days. |
| [178] |
| (7) C57BL/6 mice were fed with an HFD containing procyanidin B2 (0.2% w:w) for 8 weeks. |
| [179] |
| (8) C56BL/6J mice were fed with an HFD and administered with PAC isolated from bayberry leaves (100 mg/kg bw/day) for 8 weeks. |
| [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V. Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction. Molecules 2024, 29, 709. https://doi.org/10.3390/molecules29030709
Thilakarathna WPDW, Rupasinghe HPV. Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction. Molecules. 2024; 29(3):709. https://doi.org/10.3390/molecules29030709
Chicago/Turabian StyleThilakarathna, Wasitha P. D. W., and H. P. Vasantha Rupasinghe. 2024. "Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction" Molecules 29, no. 3: 709. https://doi.org/10.3390/molecules29030709
APA StyleThilakarathna, W. P. D. W., & Rupasinghe, H. P. V. (2024). Proanthocyanidins-Based Synbiotics as a Novel Strategy for Nonalcoholic Fatty Liver Disease (NAFLD) Risk Reduction. Molecules, 29(3), 709. https://doi.org/10.3390/molecules29030709