Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic from Simulated Wastewater Using a Metal Organic Framework
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of the Synthesized MOF-5
2.1.1. PXRD Analysis
2.1.2. FT-IR Analysis
2.1.3. FE-SEM Analysis
2.1.4. EDS Analysis
2.2. Effect of pH
2.3. Effect of Adsorbent Dose
2.4. Effect of Contact Time and Adsorption Kinetics
2.5. Effect of Adsorbate Dosage and Isotherm Modeling
2.6. Thermodynamics of Adsorption
2.7. Effect of Natural Ions and Molecules Present in Real Water
2.8. Regeneration of Adsorbent
2.9. Plausible Mechanism of Adsorption
3. Materials and Methods
3.1. Chemicals and Reagents
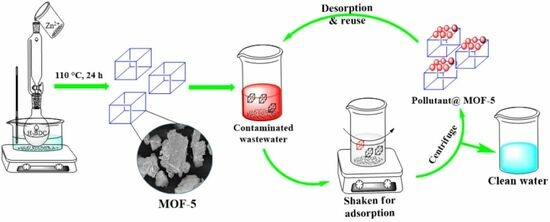
3.2. Preparation of MOF-5
3.3. Characterization Techniques
3.4. Adsorption Isotherm Model
3.5. Adsorption Thermodynamics
4. Experimental
4.1. Batch Adsorption Experiments
4.2. Regeneration of Adsorbent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maheshwari, K.; Solanki, Y.S.; Ridoy, M.S.H.; Agarwal, M.; Dohare, R.; Gupta, R. Ultrasonic Treatment of Textile Dye Effluent Utilizing Microwave-assisted Activated Carbon. Environ. Prog. Sustain. Energy 2020, 39, e13410. [Google Scholar] [CrossRef]
- Arif, A. Water Pollution and Industries. Pure Appl. Biol. 2020, 9, 2214–2224. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial Decolorization of Textile-Dyecontaining Effluents: A Review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Winckler, J. Vitalfärbung von Lysosomen und anderen Zellorganellen der Ratte mit Neutralrot Vital Staining of Lysosomes and Other Cell Organelles of the Rat with Neutral Red. Prog. Histochem. Cytochem. 1974, 6, 1–89. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Pinheiro, N.S.; Perez-Lopez, O.W.; Gutterres, M. Solid Leather Wastes as Adsorbents for Cationic and Anionic Dye Removal. Environ. Technol. 2022, 43, 1285–1293. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Wall, A.; Board, T. Chemical Basis for the Histological Use of Safranin O in the Study of Articular Cartilage. In Classic Papers in Orthopaedics; Banaszkiewicz, P.A., Kader, D.F., Eds.; Springer: London, UK, 2014; pp. 433–435. ISBN 978-1-4471-5450-1. [Google Scholar]
- Sharifpour, E.; Ghaedi, M.; Nasiri Azad, F.; Dashtian, K.; Hadadi, H.; Purkait, M.K. Zinc Oxide Nanorod-loaded Activated Carbon for Ultrasound-assisted Adsorption of Safranin O: Central Composite Design and Genetic Algorithm Optimization. Appl. Organomet. Chem. 2018, 32, e4099. [Google Scholar] [CrossRef]
- Aronson, J.K. Mercury and Mercurial Salts. In Meyler’s Side Effects of Drugs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 844–852. ISBN 978-0-444-53716-4. [Google Scholar]
- Hill, S.L.; Thomas, S.H.L. Clinical Toxicology of Newer Recreational Drugs. Clin. Toxicol. 2011, 49, 705–719. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Porter, C.J.; Cheng, W.; Zhang, X.; Wang, L.; Elimelech, M. Concentration and Recovery of Dyes from Textile Wastewater Using a Self-Standing, Support-Free Forward Osmosis Membrane. Environ. Sci. Technol. 2019, 53, 3078–3086. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, D.; Wu, Y.; Zhou, P.; Lan, Y. Degradation of Methyl Orange by Zn(0) Assisted with Silica Gel. J. Hazard. Mater. 2011, 194, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Cui, T.; Huang, F.; Zhang, Y.; Han, W. Membrane Separation Coupled with Electrochemical Advanced Oxidation Processes for Organic Wastewater Treatment: A Short Review. Membranes 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Nachimuthu, P.; Awasthi, M.K.; Ravindran, B.; Chang, S.W.; Palanichamy, M.; Nguyen, D.D. Application of Electrochemical Treatment for the Removal of Triazine Dye Using Aluminium Electrodes. J. Water Supply Res. Technol. Aqua 2020, 69, 345–354. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Ghosh, N.N.; Pulhani, V.; Biswas, G.; Singhal, P. Bio-Functionalized Magnetic Nanoparticles for Cost-Effective Adsorption of U(VI): Experimental and Theoretical Investigation. RSC Adv. 2023, 13, 15015–15023. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of Iron Oxide Nanomaterials in Wastewater Treatment: A Review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Das, C.; Sen, S.; Singh, T.; Ghosh, T.; Paul, S.S.; Kim, T.W.; Jeon, S.; Maiti, D.K.; Im, J.; Biswas, G. Green Synthesis, Characterization and Application of Natural Product Coated Magnetite Nanoparticles for Wastewater Treatment. Nanomaterials 2020, 10, 1615. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Rani, U.A.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E. A Review of Carbon Quantum Dots and Their Applications in Wastewater Treatment. Adv. Colloid Interface Sci. 2020, 278, 102124. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of Metal Organic Framework in Wastewater Treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Aslam, A.A.; Irshad, A.; Nazir, M.S.; Atif, M. A Review on Covalent Organic Frameworks as Adsorbents for Organic Pollutants. J. Clean. Prod. 2023, 400, 136737. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Tan, Y.-X.; He, Y.-P.; Wang, M.; Zhang, J. A Water-Stable Zeolite-like Metal–Organic Framework for Selective Separation of Organic Dyes. RSC Adv. 2014, 4, 1480–1483. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective Adsorption of Cationic Dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of Water Stable Metal–Organic Frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive Removal of Hazardous Materials Using Metal-Organic Frameworks (MOFs): A Review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.C.; Upadhyay, S.N. Removal of a Cationic Dye from Wastewaters by Adsorption on Activated Carbon Developed from Coconut Coir. Energy Fuels 2009, 23, 2983–2988. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Karagoz, S.; Tay, T.; Ucar, S.; Erdem, M. Activated Carbons from Waste Biomass by Sulfuric Acid Activation and Their Use on Methylene Blue Adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef]
- Shokry Hassan, H.; Elkady, M.F.; El-Shazly, A.H.; Bamufleh, H.S. Formulation of Synthesized Zinc Oxide Nanopowder into Hybrid Beads for Dye Separation. J. Nanomater. 2014, 2014, 967492. [Google Scholar] [CrossRef]
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.N. Adsorption of Malachite Green Dye from Aqueous Solutions Using Mesoporous Chitosan–Zinc Oxide Composite Material. Environ. Chem. Ecotoxicol. 2020, 2, 115–125. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Synthesis, Characterization, and Regeneration of an Inorganic–Organic Nanocomposite (ZnO@biomass) and Its Application in the Capture of Cationic Dye. Sci. Rep. 2020, 10, 14441. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. The Pioneering Role of Metal–Organic Framework-5 in Ever-Growing Contemporary Applications—A Review. RSC Adv. 2022, 12, 14282–14298. [Google Scholar] [CrossRef]
- Greer, H.F.; Liu, Y.; Greenaway, A.; Wright, P.A.; Zhou, W. Synthesis and Formation Mechanism of Textured MOF-5. Cryst. Growth Des. 2016, 16, 2104–2111. [Google Scholar] [CrossRef]
- Biserčić, M.S.; Marjanović, B.; Vasiljević, B.N.; Mentus, S.; Zasońska, B.A.; Ćirić-Marjanović, G. The Quest for Optimal Water Quantity in the Synthesis of Metal-Organic Framework MOF-5. Microporous Mesoporous Mater. 2019, 278, 23–29. [Google Scholar] [CrossRef]
- Barthwal, S.; Jeon, Y.; Lim, S.-H. Superhydrophobic Sponge Decorated with Hydrophobic MOF-5 Nanocoating for Efficient Oil-Water Separation and Antibacterial Applications. Sustain. Mater. Technol. 2022, 33, e00492. [Google Scholar] [CrossRef]
- Wang, S.; Xie, X.; Xia, W.; Cui, J.; Zhang, S.; Du, X. Study on the Structure Activity Relationship of the Crystal MOF-5 Synthesis, Thermal Stability and N2 Adsorption Property. High Temp. Mater. Process. 2020, 39, 171–177. [Google Scholar] [CrossRef]
- Wang, S.; Ye, B.; An, C.; Wang, J.; Li, Q.; Guo, H.; Zhang, J. Exploring the Coordination Effect of GO@MOF-5 as Catalyst on Thermal Decomposition of Ammonium Perchlorate. Nanoscale Res. Lett. 2019, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, N.; Kumar, M.G.; Rajan, K.S.; Rayappan, J.B.B. Metal Organic Framework (MOF-5) For Sensing of Volatile Organic Compounds. J. Appl. Sci. 2012, 12, 1681–1685. [Google Scholar] [CrossRef]
- Mirsoleimani-azizi, S.M.; Setoodeh, P.; Zeinali, S.; Rahimpour, M.R. Tetracycline Antibiotic Removal from Aqueous Solutions by MOF-5: Adsorption Isotherm, Kinetic and Thermodynamic Studies. J. Environ. Chem. Eng. 2018, 6, 6118–6130. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical Synthesis of MOF-5. Chem. Commun. 2008, 47, 6336–6338. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Son, W.-J.; Kim, J.; Ahn, W.-S. Metal–Organic Framework MOF-5 Prepared by Microwave Heating: Factors to Be Considered. Microporous Mesoporous Mater. 2008, 116, 727–731. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, Y.S. Adsorption and Diffusion of Carbon Dioxide on Metal−Organic Framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Elsherbiny, A.S.; Rady, A.; Abdelhameed, R.M.; Gemeay, A.H. Efficiency and Selectivity of Cost-Effective Zn-MOF for Dye Removal, Kinetic and Thermodynamic Approach. Environ. Sci. Pollut. Res. 2023, 30, 106860–106875. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Yao, W.; Wang, X.; Yu, S.; Yu, Z.; Wang, X. Synthesis of Rod-like Metal-Organic Framework (MOF-5) Nanomaterial for Efficient Removal of U(VI): Batch Experiments and Spectroscopy Study. Sci. Bull. 2018, 63, 831–839. [Google Scholar] [CrossRef]
- Tomić, N.M.; Dohčević-Mitrović, Z.D.; Paunović, N.M.; Mijin, D.Ž.; Radić, N.D.; Grbić, B.V.; Aškrabić, S.M.; Babić, B.M.; Bajuk-Bogdanović, D.V. Nanocrystalline CeO 2−δ as Effective Adsorbent of Azo Dyes. Langmuir 2014, 30, 11582–11590. [Google Scholar] [CrossRef]
- Ghoohestani, E.; Samari, F.; Homaei, A.; Yosuefinejad, S. A Facile Strategy for Preparation of Fe3O4 Magnetic Nanoparticles Using Cordia Myxa Leaf Extract and Investigating Its Adsorption Activity in Dye Removal. Sci. Rep. 2024, 14, 84. [Google Scholar] [CrossRef]
- Di, J.; Ruan, Z.; Zhang, S.; Dong, Y.; Fu, S.; Li, H.; Jiang, G. Adsorption Behaviors and Mechanisms of Cu2+, Zn2+ and Pb2+ by Magnetically Modified Lignite. Sci. Rep. 2022, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtaseb, A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Kinetic and Thermodynamics of Chromium Ions Adsorption onto Low-Cost Dolomite Adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Dahlan, I.; Wan Mazlan, W.H.; Mulkan, A.; Zwain, H.M.; Hassan, S.R.; Aziz, H.A.; Hasan, H.Y.A.; Zekker, I. Modeling of Batch Organic Dye Adsorption Using Modified Metal-Organic Framework-5. Chem. Eng. Technol. 2022, 45, 2080–2087. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Moghanlo, S.; Kazemi, M.S.; Nazari, S.; Ghadiri, S.K.; Saleh, H.N.; Sillanpää, M. Comparative Removal of Hazardous Cationic Dyes by MOF-5 and Modified Graphene Oxide. Sci. Rep. 2022, 12, 15314. [Google Scholar] [CrossRef]
- Iprahim, M.; Farag, R.; Tantawe, H. Removal of Dyes from Aqueous Solution Using Zinc Terephthalic Acid Metal-organic Frameworks (Zn-TPA-MOF) Based Adsorbent. Egypt. J. Chem. 2022, 66, 295–305. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and Kinetic Studies on Methylene Blue Adsorption by Coir Pith Carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Al-Rashed, S.M.; Al-Gaid, A.A. Kinetic and Thermodynamic Studies on the Adsorption Behavior of Rhodamine B Dye on Duolite C-20 Resin. J. Saudi Chem. Soc. 2012, 16, 209–215. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Luo, J.; Zou, C.; Yang, Y.; Yang, S. Selective Adsorption of Cationic Dyes from Aqueous Solution by Polyoxometalate-Based Metal–Organic Framework Composite. Appl. Surf. Sci. 2016, 362, 517–524. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Y.; Li, M.; Wu, Q.; Wang, C.; Wang, Z. Synthesis of Magnetic Nanoporous Carbon from Metal-Organic Framework for the Fast Removal of Organic Dye from Aqueous Solution. J. Magn. Magn. Mater. 2016, 407, 24–30. [Google Scholar] [CrossRef]
- Budavari, S. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Centennial ed.; Merck: Rahway, NJ, USA, 1989; ISBN 978-0-911910-28-5. [Google Scholar]
- Tong, M.; Liu, D.; Yang, Q.; Devautour-Vinot, S.; Maurin, G.; Zhong, C. Influence of Framework Metal Ions on the Dye Capture Behavior of MIL-100 (Fe, Cr) MOF Type Solids. J. Mater. Chem. A 2013, 1, 8534. [Google Scholar] [CrossRef]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of Metal-Organic Framework Hybrid Nanocomposites Based on GO and CNT with High Adsorption Capacity for Dye Removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Tang, Z.; Niu, H.; Cai, Y.; Meng, W.; Wu, F.; Giesy, J.P. Synthesis of Magnetic Metal-Organic Framework (MOF) for Efficient Removal of Organic Dyes from Water. Sci. Rep. 2015, 5, 11849. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs Nanocomposites for Rapid Removal of Heavy Metal Ions and Dyes from Water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Liang, Y.; Han, T.; Huang, H.; Yang, Q.; Liu, D.; Zhong, C. Rational Construction of Defects in a Metal–Organic Framework for Highly Efficient Adsorption and Separation of Dyes. Chem. Eng. J. 2016, 289, 486–493. [Google Scholar] [CrossRef]
- Yao, S.; Xu, T.; Zhao, N.; Zhang, L.; Huo, Q.; Liu, Y. An Anionic Metal–Organic Framework with Ternary Building Units for Rapid and Selective Adsorption of Dyes. Dalton Trans. 2017, 46, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Van Hecke, K.; Khattak, Z.A.K.; Gaoke, Z.; Danish, M.; Verpoort, F. Synthesis of 2D MOF Having Potential for Efficient Dye Adsorption and Catalytic Applications. Catal. Sci. Technol. 2018, 8, 4010–4017. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yan, X.-P. Metal–Organic Framework MIL-100(Fe) for the Adsorption of Malachite Green from Aqueous Solution. J. Mater. Chem. 2012, 22, 7449. [Google Scholar] [CrossRef]
- Chand, S.; Elahi, S.M.; Pal, A.; Das, M.C. A New Set of Cd(II)-Coordination Polymers with Mixed Ligands of Dicarboxylate and Pyridyl Substituted Diaminotriazine: Selective Sorption towards CO2 and Cationic Dyes. Dalton Trans. 2017, 46, 9901–9911. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Removal of Congo Red Dye from Aqueous Solution with Nickel-Based Metal-Organic Framework/Graphene Oxide Composites Prepared by Ultrasonic Wave-Assisted Ball Milling. Ultrason. Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef]
- Hazrati, M.; Safari, M. Cadmium-based Metal–Organic Framework for Removal of Dye from Aqueous Solution. Environ. Prog. Sustain. Energy 2020, 39, e13411. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and Enhanced Hydrostability and Hydrogen Storage Capacity of CNT@MOF-5 Hybrid Composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Yousef, N.S.; Farouq, R.; Hazzaa, R. Adsorption Kinetics and Isotherms for the Removal of Nickel Ions from Aqueous Solutions by an Ion-Exchange Resin: Application of Two and Three Parameter Isotherm Models. Desalination Water Treat. 2016, 57, 21925–21938. [Google Scholar] [CrossRef]
- Lin, K.; Pan, J.; Chen, Y.; Cheng, R.; Xu, X. Study the Adsorption of Phenol from Aqueous Solution on Hydroxyapatite Nanopowders. J. Hazard. Mater. 2009, 161, 231–240. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Yılmaz, M.; Ragab, S.; Hassaan, M.A.; El Nemr, A. Isotherm and Kinetic Studies of Acid Yellow 11 Dye Adsorption from Wastewater Using Pisum Sativum Peels Microporous Activated Carbon. Sci. Rep. 2023, 13, 4268. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.-J. Biosorption Isotherms, Kinetics and Thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Anastopoulos, I. Response to “Some Remarks on a Critical Review of the Estimation of the Thermodynamic Parameters on Adsorption Equilibria. Wrong Use of Equilibrium Constant in the van’t Hoff Equation for Calculation of Thermodynamic Parameters of Adsorption. J. Mol. Liq. 2019, 273, 425–434”. J. Mol. Liq. 2019, 280, 298–300. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H. Equilibrium, Thermodynamics and Mechanisms of Ni2+ Biosorption by Aerobic Granules. Biochem. Eng. J. 2007, 35, 174–182. [Google Scholar] [CrossRef]
- Garcia-Delgado, R.A.; Cotoruelo-Minguez, L.M.; Rodriguez, J.J. Equilibrium Study of Single-Solute Adsorption of Anionic Surfactants with Polymeric XAD Resins. Sep. Sci. Technol. 1992, 27, 975–987. [Google Scholar] [CrossRef]
- Das, C.; Singh, S.; Bhakta, S.; Mishra, P.; Biswas, G. Bio-Modified Magnetic Nanoparticles with Terminalia Arjuna Bark Extract for the Removal of Methylene Blue and Lead (II) from Simulated Wastewater. Chemosphere 2022, 291, 132673. [Google Scholar] [CrossRef] [PubMed]







| Adsorbate | Type of Dye | Qmax (mg/g) | References |
|---|---|---|---|
| Aniline blue | Cationic | 55.34 | [49] |
| Methylene blue | Cationic | 51.81 | [55] |
| Malachite green | Cationic | 50.69 | [56] |
| Methylene blue | Cationic | 2.632 | [49] |
| Safranin O | Cationic | 8.19 | This study |
| Congo Red | Anionic | 769.23 | [57] |
| Orange II | Anionic | 10.01 | [49] |
| Merbromin | Anionic | 22.22 | This study |
| Adsorbent | Adsorbate | Qmax (mg/g) | References |
|---|---|---|---|
| Coconut coir | Methylene blue | 15.59 | [32] |
| Egg shell | Methylene blue | 16.43 | [33] |
| Activated carbon from waste biomass | Methylene blue | 10.21 | [34] |
| ZnO hydrid beads | Basic blue 41 | 1.0–8.0 | [35] |
| Polylactide/spent grain | Malachite green | 1.48 | [36] |
| ZnO nanoparticles | Methylene blue | 0.3428 | [37] |
| Coir pith carbon | Methylene blue | 5.87 | [58] |
| ZnO@ananas comosus waste biomass | Celestine blue | 6.52 | [37] |
| Ananas comosus waste biomass | Celestine blue | 5.42 | [37] |
| Brewery spent grain | Malachite green | 2.55 | [36] |
| Poultry feathers | Malachite green | 3.55 | [36] |
| Ananas comosus waste biomass | Celestine blue | 5.42 | [37] |
| MOF-5 | Merbromin | 22.22 | This study |
| MOF-5 | Safranin O | 8.19 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, N.; Das, C.; Paul, M.; Im, J.; Biswas, G. Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic from Simulated Wastewater Using a Metal Organic Framework. Molecules 2024, 29, 886. https://doi.org/10.3390/molecules29040886
Roy N, Das C, Paul M, Im J, Biswas G. Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic from Simulated Wastewater Using a Metal Organic Framework. Molecules. 2024; 29(4):886. https://doi.org/10.3390/molecules29040886
Chicago/Turabian StyleRoy, Nilanjan, Chanchal Das, Mohuya Paul, Jungkyun Im, and Goutam Biswas. 2024. "Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic from Simulated Wastewater Using a Metal Organic Framework" Molecules 29, no. 4: 886. https://doi.org/10.3390/molecules29040886
APA StyleRoy, N., Das, C., Paul, M., Im, J., & Biswas, G. (2024). Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic from Simulated Wastewater Using a Metal Organic Framework. Molecules, 29(4), 886. https://doi.org/10.3390/molecules29040886