Reaction of Partially Methylated Polygalacturonic Acid with Iron(III) Chloride and Characterization of a New Mixed Chloride–Polygalacturonate Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Preparation and Characterization of Compounds 1 and 2
2.1.1. Hydrolysis of Pectin
2.1.2. The Preparation of Compound 1 with the Use of Double Ion Exchange
Polymer—SO3Na
2.1.3. The Preparation of Compound 2
2.2. Spectroscopic Characterization of Compound 2
2.2.1. Mössbauer Spectroscopic Results of Compound 2
2.2.2. XPS Results of Compound 2
2.2.3. ESR Results of Compound 2
2.2.4. Magnetic Measurements on Compound 2
2.2.5. IR Spectroscopy of Compound 2
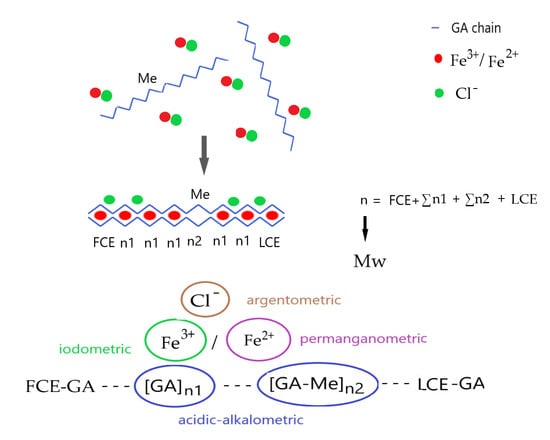
2.3. Determining the Polymerization Degree (Average Molecular Weight) of Polygalacturonic Acid and Its Fe(III) Salt
- (1)
- Contrary to the free D-galacturonic acid FeIII complex, compound 1 cannot completely neutralize all three charges of FeIII, and compound 2 that is formed contains a foreign counter ion (chloride due to the use of FeCl3) as well (Table S2).
- (2)
- The non-demethylated GA units (GA-Me from the incomplete hydrolysis of pectin) in compound 1 do not provide any charge increment to neutralize the excess charge of FeIII ions in the FeIII(µ-O)(µ-OH)FeIII core.
3. Materials and Methods
4. Conclusions
- (1)
- We developed a mild method to prepare polygalacturonic acid (compound 1) with the partial de-esterification of pectin followed by a double-ion-exchange process with the use of a styrene–divinylbenzene copolymer-based sulfonated macroreticular ion-exchanger resin. The reaction of compound 1 with FeCl3 resulted in a basic PGA-iron(III) complex: compound 2. The complex has a polymeric nature with ~1:2 Fe:GA stoichiometry and contains outer-sphere chloride ions.
- (2)
- Compound 2 contains two different and distorted FeIII octahedral centers in FeIII(µ-O)(µ-OH)FeIII units due to the asymmetrical ligation by two ionic carboxylates and two bidentate-bridging GA units containing methylated carboxylate with bidentate-chelating and C=O…Fe/glycosidic O…Fe coordination modes, respectively. The bridging ligands connect the neighboring Fe(µ-O)(µ-OH)Fe dimeric units into an egg-box-like polymeric structure. Two outer-sphere chloride anions are fixed in different environments by hydrogen bonds.
- (3)
- FeIII was partially reduced into FeII in the reaction of compound 1 and FeCl3 due to the ring-opening of the chain-end galacturonic acid units of compound 1. This reaction ensures an easy route to determine the number of polymer chains, the average polymerization degree, and accordingly, the average molecular weight of polygalacturonic acid (PGA) and its FeIII salt (compounds 1 and 2). The amount of FeII from the redox reaction of FeIII and compound 1 is proportional to the total number of chain ends of polygalacturonic acid units. The ratio of overall galacturonic acid (free and methylated) and chain-end galacturonic acid gives the average polymerization degree of compounds 1 and 2. The number of galacturonic acid units was determined both from CHN analysis and from Fe and chloride content with the use of the charge neutrality principle. The average degree of polymerization was n = 211 and 212 from the CHN analysis and titrimetric routes, respectively. The average molecular weight of the tested commercial polygalacturonic acid was ~50,000 g/mol.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minzanova, S.T.; Khabibullina, A.V.; Arkhipova, D.M.; Mironova, L.G.; Vyshtakalyuk, A.B.; Kholin, K.V.; Zakirova, Y.M.; Zakirova, G.S.; Semenov, E.I.; Milyukov, V.A.; et al. Anti-anemic activity of sodium, calcium, iron-Polygalacturonate in vivo in rabbits. BioNanoScience 2022, 2, 170–183. [Google Scholar] [CrossRef]
- du Poset, M.A.; Lerbret, A.; Zitolo, A.; Cousin, F.; Assifaoui, A. Design of polygalacturonate hydrogels using iron(II) as cross-linkers: A promising route to protect bioavailable iron against oxidation. Carbohyd. Polym. 2018, 188, 276–283. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Khamatgalimov, A.R.; Ryzhkina, I.S.; Murtazina, L.I.; Mironova, L.G.; Kadirov, M.K.; Mironov, V.F. Synthesis and physicochemical properties of antianemic iron and calcium complexes with sodium polygalacturonate. Dokl. Phys. Chem. 2016, 467, 45–48. [Google Scholar] [CrossRef]
- Vyshtakaliuk, A.B.; Zobov, V.V.; Minzanova, S.T.; Lantsova, A.V.; Mironov, V.F.; Petrova, G.R.; Konovalov, A.I. Antianemic activity of water-soluble Na,Ca,Fe-polygalacturonate. Bull. Exp. Biol. Med. 2010, 150, 45–47. [Google Scholar] [CrossRef]
- Lakatos, B.; Szentmihályi, K.; Vinkler, P.; Balla, J.; Balla, G. The role of essential metal ions in the human organism and their oral supplementation to the human body in deficiency states. Orvosi Hetil. 2004, 145, 1315–1319. [Google Scholar]
- Fodor, J.; May, Z.; Hajdú, M.; Kótai, L.; Szentmihályi, K. Examination of iron binding capacity of polygalacturonic acid and release of iron from the iron-polygalacturonate complex. In Trace Elements in the Food Chain, Proceedings of the an International Symposium on Trace Elements in the Food Chain, Budapest, Hungary, 25–27 May 2006; Working Committee on Trace Elements of the Complex Committee Hungarian Academy of Sciences (HAS): Budapest, Hungary, 2006; pp. 102–106. [Google Scholar]
- Minzanova, S.T.; Chekunkov, E.V.; Khabibullina, A.V.; Vyshtakalyuk, A.B.; Kholin, K.V.; Mironova, L.G.; Nizameeva, G.R.; Khamatgalimov, A.R.; Ryzhkina, I.S.; Murtazina, L.I.; et al. A new pharmacological composition based on water-soluble pectin metal compelxes stimulating hematopoiesis. Russ. Chem. Bull. 2023, 72, 2263–2277. [Google Scholar] [CrossRef]
- Wang, T.; Tao, Y.H.; Lai, C.H.; Huang, C.X.; Ling, Z.; Yong, Q. A method for quantitative characterization of incomplete degradation products of polygalacturonic acid. Int. J. Biol. Macromol. 2021, 188, 343–349. [Google Scholar] [CrossRef] [PubMed]
- White, G.W.; Katona, T.; Zodda, J.P. The use of high-performance size exclusion chromatography (HPSEC) as a molecular weight screening technique for polygalacturonic acid for use in pharmaceutical applications. J. Pharm. Biomed. Anal. 1999, 20, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crepeau, M.J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Gupta, D.; Jassal, M.; Agrawal, A.K. Solution properties and electrospinning of poly(galacturonic acid) nanofibers. Carbohydr. Polym. 2019, 212, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, M.Y.; Tang, S.; Lai, C.H.; Huang, C.X.; Fan, Y.M.; Yong, Q. Preparation of di- and trigalacturonic acid by coupling hydrothermal pretreatment and enzymatic hydrolysis. Process Biochem. 2021, 102, 180–185. [Google Scholar] [CrossRef]
- Hourdet, D.; Muller, G. Solution properties of pectin polysaccharides II. Conformation and molecular size of high galacturonic acid content isolated pectin chains. Carbohydr. Polym. 1991, 16, 113–135. [Google Scholar] [CrossRef]
- Rinaudo, M.; Ravanat, G. NMR investigation on oligo- and poly(ga1acturonic acid)s; gel formation in the presence of Ca2+ counterions. Makromol. Chem. 1980, 181, 1059–1070. [Google Scholar] [CrossRef]
- Kertesz, Z.I. The Pectic Substances; Interscience Publishers Inc.: New York, NY, USA; London, UK, 1951. [Google Scholar]
- Dengel-Szentmihályi, K.; Lakatos, B.; Vinkler, P.; Kótai, L.; Sándor, Z.; Biró, P. Metal Complexes of Polygalacturonic Acid and Its Production. HU227595B1, 7 August 2003. [Google Scholar]
- Micera, G.; Deiana, S.; Gessa, C.; Petrera, M. Spectroscopic studies on the binding of iron (II) and (III) ions to polygalacturonic acid. Inorg. Chim. Acta 1981, 56, 109–113. [Google Scholar] [CrossRef]
- Gessa, C.; De Cherchi, M.L.; Dessi, A.; Deiana, S.; Micera, G. The reduction of Fe(III) to Fe(II) and V(V) to V(IV) by polygalacturonic acid:a reduction and complexation mechanism of biochemical significance. Inorg. Chim. Acta 1983, 80, L53–L55. [Google Scholar] [CrossRef]
- Lakatos, B.; Korecz, L.; Meisel, J. Comparative study on the Mossbauer parameters of iron humate and polyuronates. Geoderma 1977, 19, 149–157. [Google Scholar] [CrossRef]
- Kiyama, M.; Takada, T. The hydrolysis of ferric complexes. Magnetic and spectrophotometric studies of aqueous solutions of ferric salts. Bull. Chem. Soc. Jpn. 1973, 46, 1680–1686. [Google Scholar] [CrossRef]
- [21 Lakatos, B.; Holly, S.; Meisel, J. Infrared spectroscopic study on metal pectates. Inorg. Chim. Acta 1983, 79, 270–271. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Part A and B.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ferraro, J.R. Low-Frequency Vibrations of Inorganic and Coordination Compounds; Pergamon Press: New York, NY, USA, 1971. [Google Scholar]
- du Poset, A.M.; Zitolo, A.; Cousin, F.; Assifaoui, A.; Lerbret, A. Evidence for an egg-box-like structure in iron(II)-polygalacturonate hydrogels: A combined EXAFS and molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2020, 22, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Mády, G.; Lakatos, B.; Kever, J.J.; Yakonova, N.V.D. Determination of the molecular mass of polygalacturonic acid. Acta Aliment. 1992, 21, 337–349. [Google Scholar]
- Deiana, S.; Gessa, C.; Solinas, V.; Piu, P.; Seeber, R. Complexing and redox properties of the system D-galacturonic acid-iron (III). J. Inorg. Biochem. 1989, 35, 107–113. [Google Scholar] [CrossRef]
- Deiana, S.; Gessa, C.; Manunza, B.; Piu, P.; Seeber, R. Iron(III) reduction by D-galacturonic acid. Part I. Influence of copper (II) complexes formation. J. Inorg. Biochem. 1990, 39, 25–32. [Google Scholar] [CrossRef]
- Deiana, S.; Gessa, C.; Solinas, V.; Piu, P.; Seeber, R. Analytical study of the interactions of d-galacturonic acid with iron(III) and iron(II) in solution and with iron(III)-bentonite. Anal. Chim. Acta 1989, 222, 315–322. [Google Scholar] [CrossRef]
- Deiana, S.; Gessa, C.; Piu, P.; Seeber, R. Iron (III) reduction by D-galacturonic acid. Part II. Influence of uranyl(VI), lead(II), nickel(II), and cadmium(II) complexes formation. J. Inorg. Biochem. 1990, 40, 301–307. [Google Scholar] [CrossRef]
- Rudan-Tasic, D.; Klofutar, C. Potentiometric titration of poly(α-D)galacturonic acid. In Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 609–617. [Google Scholar]
- Erdey, L. Introduction to Chemical Analysis, 8th ed.; Tankönyvkiadó: Budapest, Hungary, 1963; Volume II. [Google Scholar]
- Rahbar, M.; Paull, B.; Macka, M. Instrument-free argentometric determination of chloride via trapezoidal distance-based microfluidic paper devices. Anal. Chim. Acta 2019, 1063, 1–8. [Google Scholar] [CrossRef]
- Sajo, I.E.; Kótai, L.; Keresztury, G.; Gács, I.; Pokol, G.; Kristóf, J.; Soptrayanov, B.; Petrusevski, V.M.; Timpu, D.; Sharma, P.K. Studies on the Chemistry of Tetraamminezinc(II) Dipermanganate ([Zn(NH3)4](MnO4)2): Low-Temperature Synthesis of the Manganese Zinc Oxide (ZnMn2O4) Catalyst Precursor. Helv. Chim. Acta 2008, 91, 1646–1658. [Google Scholar] [CrossRef]
- Kótai, L.; Banerji, K.K.; Sajó, I.; Kristóf, J.; Sreedhar, B.; Holly, S.; Keresztury, G.; Rockenbauer, A. An Unprecedented-Type Intramolecular Redox Reaction of Solid Tetraamminecopper(2+) Bis(permanganate) ([Cu(NH3)4](MnO4)2)—A Low-Temperature Synthesis of Copper Dimanganese Tetraoxide-Type (CuMn2O4) Nanocrystalline Catalyst Precursors. Helv. Chim. Acta 2002, 85, 2316–2327. [Google Scholar] [CrossRef]
- Kotai, L.; Argay, G.; Holly, S.; Keszler, A.; Pukanszky, B.; Banerji, K.K. Study on the Existence of Hydrogen Bonds in ammonium permanganate. Z. Anorg. Allg. Chem. 2001, 627, 114–118. [Google Scholar] [CrossRef]
- Kazinczy, B.; Kotai, L.; Sajo, I.E.; Holly, S.; Lazar, K.; Jakab, E.; Gacs, E.; Szentmihalyi, K. Phase Relations and Heat-Induced Chemical Processes in Sludges of Hot-Dip Galvanization. Ind. Eng. Chem. Res. 2002, 41, 720–725. [Google Scholar] [CrossRef]







| Compound | Component | IS, mm/s | QS, mm/s | FWHM, mm/s | RI |
|---|---|---|---|---|---|
| PGA-FeIII | FeIII(a) | 0.40 | 0.62 | 0.44 | 45 |
| FeIII(b) | 0.42 | 1.10 | 0.57 | 38 | |
| FeII(a) | 0.97 | 2.16 | 0.42 | 5 | |
| FeII(b) | 1.31 | 2.28 | 0.55 | 12 |
| Analytical Method | n (Pieces) | Average Molecular Weight, g/mol |
|---|---|---|
| Light scattering | 242 | Mw = 47,500 |
| Osmometric method | 249 | Mn = 48,735 |
| Viscosity measurement | 239 | Mw = 46,933 |
| Titrimetry + CHN analysis | 255 | Mn = 50,156 |
| Titrimetry with chloride analysis | 254 | Mn = 49,920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kótai, L.; Lázár, K.; Kiss, L.F.; Szentmihályi, K. Reaction of Partially Methylated Polygalacturonic Acid with Iron(III) Chloride and Characterization of a New Mixed Chloride–Polygalacturonate Complex. Molecules 2024, 29, 890. https://doi.org/10.3390/molecules29040890
Kótai L, Lázár K, Kiss LF, Szentmihályi K. Reaction of Partially Methylated Polygalacturonic Acid with Iron(III) Chloride and Characterization of a New Mixed Chloride–Polygalacturonate Complex. Molecules. 2024; 29(4):890. https://doi.org/10.3390/molecules29040890
Chicago/Turabian StyleKótai, László, Károly Lázár, László Ferenc Kiss, and Klára Szentmihályi. 2024. "Reaction of Partially Methylated Polygalacturonic Acid with Iron(III) Chloride and Characterization of a New Mixed Chloride–Polygalacturonate Complex" Molecules 29, no. 4: 890. https://doi.org/10.3390/molecules29040890
APA StyleKótai, L., Lázár, K., Kiss, L. F., & Szentmihályi, K. (2024). Reaction of Partially Methylated Polygalacturonic Acid with Iron(III) Chloride and Characterization of a New Mixed Chloride–Polygalacturonate Complex. Molecules, 29(4), 890. https://doi.org/10.3390/molecules29040890