Heterogeneous Brønsted Catalysis in the Solvent-Free and Multigram-Scale Synthesis of Polyalcohol Acrylates: The Case Study of Trimethylolpropane Triacrylate
Abstract
:1. Introduction
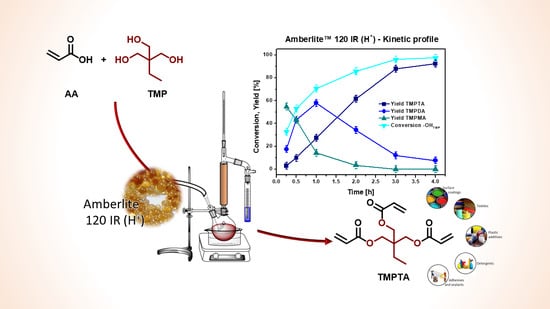
2. Results and Discussion
Scale-Up System with a Mechanical Stirrer
3. Materials and Methods
3.1. Catalytic Runs
3.2. Scale-Up with Mechanical Stirrer
3.3. 1H NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, X.; Meng, K.; Zhang, G.; Liu, M.; Song, Y.; Song, Z.; Yang, C. Interfacial catalysts for sustainable chemistry: Advances on atom and energy efficient glycerol conversion to acrylic acid. Green Chem. 2021, 23, 51–76. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Hermens, J.G.H.; Jensma, A.; Feringa, B.L. Highly Efficient Biobased Synthesis of Acrylic Acid. Angew. Chem. Int. Ed. 2022, 61, e202112618. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Onda, A.; Ogo, S.; Yanagisawa, K. Acrylic acid synthesis from lactic acid over hydroxyapatite catalysts with various cations and anions. Catal. Today 2014, 226, 192–197. [Google Scholar] [CrossRef]
- Bhagyashri, P.; Pratik, M.; Eswara, P. Acrylic Acid Market Outlook—2021–2030. Available online: https://www.alliedmarketresearch.com/acrylic-acid-market (accessed on 31 August 2023).
- Mori, H.; Müller, A.H.E. New polymeric architectures with (meth)acrylic acid segments. Prog. Polym. Sci. 2003, 28, 1403–1439. [Google Scholar] [CrossRef]
- Nollenberger, K.; Albers, J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 2013, 457, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, C.; Neri, G.; Santoro, A.; Fazio, E. Acrylate and Methacrylate Polymers’ Applications: Second Life with Inexpensive and Sustainable Recycling Approaches. Materials 2022, 15, 282. [Google Scholar] [CrossRef]
- Lin, X.; Liao, B.; Zhang, J.; Li, S.; Huang, J.; Pang, H. Synthesis and characterization of high-performance cross-linked polycarboxylate superplasticizers. Constr. Build. Mater. 2019, 210, 162–171. [Google Scholar] [CrossRef]
- Petrov, P.; Bozukov, M.; Burkhardt, M.; Muthukrishnan, S.; Müller, A.H.E.; Tsvetanov, C.B. Stabilization of polymeric micelles with a mixed poly(ethylene oxide)/poly(2-hydroxyethyl methacrylate) shell by formation of poly(pentaerythritol tetraacrylate) nanonetworks within the micelles. J. Mater. Chem. 2006, 16, 2192–2199. [Google Scholar] [CrossRef]
- Duffy, D.J.; Das, K.; Hsu, S.L.; Penelle, J.; Rotello, V.M.; Stidham, H.D. Binding Efficiency and Transport Properties of Molecularly Imprinted Polymer Thin Films. J. Am. Chem. Soc. 2002, 124, 8290–8296. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, S.J.; Kim, Y.-J.; Jung, K.-H. Selective Vapor Permeation Behavior of Crosslinked PAMPS Membranes. Polymers 2020, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Fertier, L.; Ibert, M.; Buffe, C.; Saint-Loup, R.; Joly-Duhamel, C.; Robin, J.J.; Giani, O. New biosourced UV curable coatings based on isosorbide. Prog. Org. Coat. 2016, 99, 393–399. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Nowicki, M.; Vuković-Kwiatkowska, I.; Nowakowska, S. Crosslinked blends of poly(lactic acid) and polyacrylates: AFM, DSC and XRD studies. J. Polym. Res. 2013, 20, 91. [Google Scholar] [CrossRef]
- Hatanaka, L.C.; Wang, Q.; Cheng, Z.; Mannan, M.S. Effect of trimethylolpropane triacrylate cross-linkages on the thermal stability and char yield of poly (methyl methacrylate) nanocomposites. Fire Saf. J. 2017, 87, 65–70. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Vediappan, V.; Kaliappa gounder, S. Synthesis and evaluation of trimethylolpropane triacrylate crosslinked superabsorbent polymers for conserving water and fertilizers. J. Appl. Polym. Sci. 2013, 129, 3793. [Google Scholar] [CrossRef]
- Melchiorre, M.; Esposito, R.; Russo, V.; Di Serio, M.; Elena Cucciolito, M.; Ruffo, F. Solvent-free direct esterification of acrylic acid with 2-ethylhexyl alcohol using simple Zn(II) catalysts. Inorganica Chim. Acta 2022, 534, 120821. [Google Scholar] [CrossRef]
- Grzesik, M.; Skrzypek, J.; Witczak, M. Kinetics of esterification of acrylic acid with C3 and C4 aliphatic alcohols in the presence of sulfuric acid as a catalyst. In Studies in Surface Science and Catalysis; Froment, G.F., Waugh, K.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 122, pp. 415–418. [Google Scholar]
- Jyoti, G.; Soni, R. Kinetics study of esterification reaction of acrylic acid with n-butanol. Mater. Today Proc. 2023, 72, 2720–2722. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Experimental and Kinetic Study of Esterification of Acrylic Acid with Ethanol Using Homogeneous Catalyst. Int. J. Chem. React. Eng. 2016, 14, 571–578. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.-L.; Park, S.-J. Synthesis of ditrimethylolpropane acrylate with low functionality for UV-curable coatings. J. Ind. Eng. Chem. 2012, 18, 1577–1581. [Google Scholar] [CrossRef]
- Sert, E.; Atalay, F.S. Esterification of Acrylic Acid with Different Alcohols Catalyzed by Zirconia Supported Tungstophosphoric Acid. Ind. Eng. Chem. Res. 2012, 51, 6666–6671. [Google Scholar] [CrossRef]
- Dupont, P.; Védrine, J.C.; Paumard, E.; Hecquet, G.; Lefebvre, F. Heteropolyacids supported on activated carbon as catalysts for the esterification of acrylic acid by butanol. Appl. Catal. A Gen. 1995, 129, 217–227. [Google Scholar] [CrossRef]
- Mekala, M.; Goli, V.R. Kinetics of esterification of methanol and acetic acid with mineral homogeneous acid catalyst. Chin. J. Chem. Eng. 2015, 23, 100–105. [Google Scholar] [CrossRef]
- Lilja, J.; Murzin, D.Y.; Salmi, T.; Aumo, J.; Mäki-Arvela, P.; Sundell, M. Esterification of different acids over heterogeneous and homogeneous catalysts and correlation with the Taft equation. J. Mol. Catal. A Chem. 2002, 182–183, 555–563. [Google Scholar] [CrossRef]
- Melchiorre, M.; Lentini, D.; Cucciolito, M.E.; Taddeo, F.; Hmoudah, M.; Di Serio, M.; Ruffo, F.; Russo, V.; Esposito, R. Sustainable Ketalization of Glycerol with Ethyl Levulinate Catalyzed by the Iron(III)-Based Metal-Organic Framework MIL-88A. Molecules 2022, 27, 7229. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; Di Serio, M.; Esposito, R.; Melchiorre, M.; Ruffo, F.; Russo, V.; Tesser, R.; Turco, R.; Vitiello, R. Catalysis for Oleochemical Platforms. Eur. J. Inorg. Chem. 2023, 26, e202200783. [Google Scholar] [CrossRef]
- Taddeo, F.; Vitiello, R.; Tesser, R.; Melchiorre, M.; Eränen, K.; Salmi, T.; Russo, V.; Di Serio, M. Nonanoic acid esterification with 2-ethylhexanol: From batch to continuous operation. Chem. Eng. J. 2022, 444, 136572. [Google Scholar] [CrossRef]
- Esposito, R.; Cucciolito, M.E.; D’Amora, A.; Di Guida, R.; Montagnaro, F.; Ruffo, F. Highly efficient iron(III) molecular catalysts for solketal production. Fuel Process. Technol. 2017, 167, 670–673. [Google Scholar] [CrossRef]
- Komoń, T.; Niewiadomski, P.; Oracz, P.; Jamróz, M.E. Esterification of acrylic acid with 2-ethylhexan-1-ol: Thermodynamic and kinetic study. Appl. Catal. A Gen. 2013, 451, 127–136. [Google Scholar] [CrossRef]
- Li, R.; Schork, F.J. Modeling of the Inhibition Mechanism of Acrylic Acid Polymerization. Ind. Eng. Chem. Res. 2006, 45, 3001–3008. [Google Scholar] [CrossRef]
- Keshipour, S.; Houshyar, M.; Fatemi, Z. Synthesis of some Polyol Esters and Diesters Catalyzed with SnO2 and Nano-SnO2. Iran. J. Chem. Chem. Eng. 2019, 38, 19–26. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Leite, M.J.L.; Marques, I.R.; Proner, M.C.; Araújo, P.H.H.; Ambrosi, A.; Di Luccio, M. Catalytically active membranes for esterification: A review. Chin. J. Chem. Eng. 2023, 53, 142–154. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.; Zhang, P.; Jiang, P.; Leng, Y. Protonic ionic liquids as efficient phase-separation catalysts for esterification of trimethylolpropane and acrylic acid. J. Mol. Liq. 2022, 360, 119403. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Zhang, P.; Leng, Y. Sulfonated phenylamine ionic liquids as efficient phase-separation catalysts for the synthesis of trimethylolpropane triacrylate. New J. Chem. 2023, 47, 13789–13796. [Google Scholar] [CrossRef]
- Shashkova, V.T.; Pevtsova, L.A.; Zapadinskii, B.I.; Sokolov, V.I.; Sister, V.G.; Ivannikova, E.M. Synthesizing the components of photopolymerizing acryl composites for production of waveguides with high transparency within telecommunication spectral regions. Theor. Found. Chem. Eng. 2012, 46, 546–551. [Google Scholar] [CrossRef]
- Nakatake, D.; Yazaki, R.; Ohshima, T. Chemoselective Transesterification of Acrylate Derivatives for Functionalized Monomer Synthesis Using a Hard Zinc Alkoxide Generation Strategy. Eur. J. Org. Chem. 2016, 2016, 3696–3699. [Google Scholar] [CrossRef]





| Conditions | Yield, % | Conversion, % 2 | ||
|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | ||
| No air flow | 25 | 54 | 16 | 60 |
| Venting | 26 | 54 | 17 | 61 |
| Bubbling | 3 | 31 | 65 | 87 |
| Conditions | Acid:Alcohol mol:mol | Yield, % | Conversion, % 2 | ||
|---|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | |||
| No air flow | 3:1 | 25 | 54 | 16 | 60 |
| No air flow | 6:1 | 15 | 55 | 31 | 72 |
| No air flow | 9:1 | 11 | 26 | 62 | 83 |
| Bubbling | 3:1 | 3 | 31 | 65 | 87 |
| Bubbling | 6:1 | <1 | 7 | 92 | 97 |
| Bubbling | 9:1 | <1 | 1 | 98 | 99 |
| Catalyst | Time, h | Yield, % | Conversion, % 2 | ||
|---|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | |||
| Dowex™ 50WX8 | 0.25 | 54 | 16 | 3.1 | 32 |
| 0.50 | 46 | 42 | 8.1 | 52 | |
| 4.00 | <1 | 29 | 71 | 90 | |
| Amberlyst® 15 | 0.25 | 49 | 37 | 4.0 | 45 |
| 0.50 | 28 | 59 | 10 | 59 | |
| 4.00 | <1 | <1 | >99 | >99 | |
| Amberlite™ 120 IR (H+) | 0.25 | 55 | 17 | 2.9 | 33 |
| 0.50 | 43 | 43 | 9.9 | 53 | |
| 4.00 | <1 | 8 | 92 | 98 | |
| Conditions | Acid:Alcohol mol:mol | Yield % | Conversion % 2 | ||
|---|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | |||
| Not pre-dried | 3:1 | 3 | 31 | 65 | 87 |
| Not pre-dried | 6:1 | <1 | 7 | 92 | 97 |
| Not pre-dried | 9:1 | <1 | 1 | 98 | 99 |
| Pre-dried | 3:1 | 6 | 34 | 61 | 85 |
| Pre-dried | 6:1 | <1 | 8 | 92 | 98 |
| Pre-dried | 9:1 | <1 | <1 | >99 | >99 |
| Loading w/wtot | Time, h | Yield % | Conversion % 2 | ||
|---|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | |||
| 0.25 | 55 | 17 | 2.9 | 33 | |
| 10% | 0.50 | 43 | 43 | 9.9 | 53 |
| 4.00 | <1 | 8 | 92 | 98 | |
| 0.25 | 36 | 6.0 | <1 | 16 | |
| 5.0% | 0.50 | 53 | 17 | 3.2 | 32 |
| 4.00 | <1 | 18 | 81 | 93 | |
| 0.25 | 22 | 1.6 | <1 | 9.0 | |
| 2.5% | 0.50 | 44 | 10 | 2.3 | 24 |
| 4.00 | <1 | 38 | 61 | 86 | |
| Conditions | Acid:Alcohol mol:mol | Yield % | Conversion % 2 | ||
|---|---|---|---|---|---|
| TMPMA | TMPDA | TMPTA | |||
| Bubbling | 3.9:1 | 2 | 72 | 25 | 73 |
| Bubbling | 4.5:1 | 9 | 52 | 38 | 76 |
| Bubbling | 6:1 | 5 | 42 | 54 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchiorre, M.; Cucciolito, M.E.; Esposito, R.; Silvestro, S.; Ruffo, F. Heterogeneous Brønsted Catalysis in the Solvent-Free and Multigram-Scale Synthesis of Polyalcohol Acrylates: The Case Study of Trimethylolpropane Triacrylate. Molecules 2024, 29, 918. https://doi.org/10.3390/molecules29040918
Melchiorre M, Cucciolito ME, Esposito R, Silvestro S, Ruffo F. Heterogeneous Brønsted Catalysis in the Solvent-Free and Multigram-Scale Synthesis of Polyalcohol Acrylates: The Case Study of Trimethylolpropane Triacrylate. Molecules. 2024; 29(4):918. https://doi.org/10.3390/molecules29040918
Chicago/Turabian StyleMelchiorre, Massimo, Maria E. Cucciolito, Roberto Esposito, Simone Silvestro, and Francesco Ruffo. 2024. "Heterogeneous Brønsted Catalysis in the Solvent-Free and Multigram-Scale Synthesis of Polyalcohol Acrylates: The Case Study of Trimethylolpropane Triacrylate" Molecules 29, no. 4: 918. https://doi.org/10.3390/molecules29040918
APA StyleMelchiorre, M., Cucciolito, M. E., Esposito, R., Silvestro, S., & Ruffo, F. (2024). Heterogeneous Brønsted Catalysis in the Solvent-Free and Multigram-Scale Synthesis of Polyalcohol Acrylates: The Case Study of Trimethylolpropane Triacrylate. Molecules, 29(4), 918. https://doi.org/10.3390/molecules29040918