Direct Population of Triplet States for Efficient Organic Afterglow through the Intra/Intermolecular Heavy-Atom Effect
Abstract
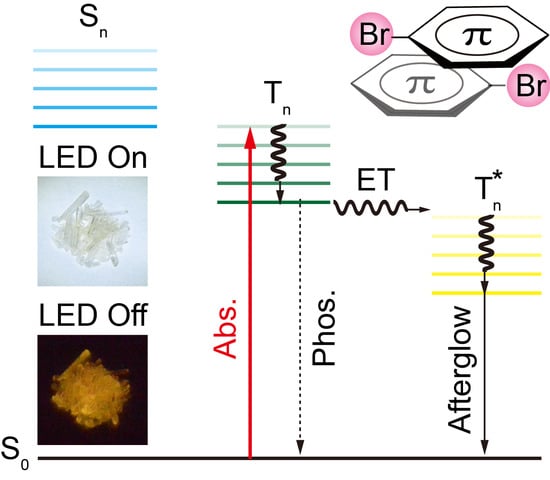
:1. Introduction
2. Results and Discussion
2.1. Molecular Design and Synthesis
2.2. Photophysical Property Investigation
2.3. Crystal Structure Analysis
2.4. Lifetime-Resolved Flexible Pattern Encryption
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Single-Crystal X-ray Analysis
3.3. Photophysical Property Investigations
3.4. Probing the Formation of Triplet Excited States
3.5. TD-DFT Calculations
3.6. Flexible Pattern Encryption Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, F.; Wang, K.-Z.; Yan, D. Supramolecular Glasses with Color-Tunable Circularly Polarized Afterglow through Evaporation-induced Self-assembly of Chiral Metal-organic Complexes. Nat. Commun. 2023, 14, 1654. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Liu, X.; Shi, Y.E.; Li, Z.; Zhao, Y. Modulating Emission of Boric Acid into Highly Efficient and Color-tunable Afterglow via Dehydration-Induced Through-Space Conjugation. Adv. Sci. 2023, 10, 2300139. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature Phosphorescence from Organic Aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Q.; Zhang, H.; Zhang, Y.; Zhan, G.; Zhang, D.; Duan, L. Enhancing the Efficiency and Stability of Blue Thermally Activated Delayed Fluorescence Emitters by Perdeuteration. Nat. Photon. 2024, 1–8. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, C.; Xiang, Y.; Wang, Z.; Xue, X.; Li, P.; Li, H.; Xie, G.; Huang, W.; Chen, R. Resonance-induced Stimuli-responsive Capacity Modulation of Organic Ultralong Room Temperature Phosphorescence. J. Am. Chem. Soc. 2022, 144, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Jinnai, K.; Kabe, R.; Lin, Z.; Adachi, C. Organic Long-persistent Luminescence Stimulated by Visible Light in p-type Systems Based On Organic Photoredox Catalyst Dopants. Nat. Mater. 2022, 21, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Xu, S.-M.; Xu, Q.; Lu, C. Large-scale Preparation for Efficient Polymer-based Room-temperature Phosphorescence via Click Chemistry. Sci. Adv. 2020, 6, eaaz6107. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Zhao, Z.; Yuan, W.Z. Clustering-triggered Phosphorescence of Nonconventional Luminophores. Sci. China Chem. 2023, 66, 367–387. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, X.; Feng, X.; Wang, Y.; Li, Z.; Lin, H. Carbon Dots with Dual-emissive, Robust, and Aggregation-induced Room-temperature Phosphorescence Characteristics. Angew. Chem. Int. Ed. 2020, 59, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Qi, Z.; Yan, D. Highly Efficient and Direct Ultralong All-phosphorescence from Metal-organic Framework Photonic Glasses. Angew. Chem. Int. Ed. 2022, 61, e202208735. [Google Scholar] [CrossRef]
- Cai, S.; Yao, X.; Ma, H.; Shi, H.; An, Z. Manipulating Intermolecular Interactions for Ultralong Organic Phosphorescence. Aggregate 2023, e320. [Google Scholar] [CrossRef]
- Shi, H.; Niu, Z.; Wang, H.; Ye, W.; Xi, K.; Huang, X.; Wang, H.; Liu, Y.; Lin, H.; Shi, H. Endowing Matrix-Free Carbon Dots with Color-tunable Ultralong Phosphorescence by Self-doping. Chem. Sci. 2022, 13, 4406–4412. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Fang, M.; Tang, B.Z.; Li, Z. Stimulus-responsive Room Temperature Phosphorescence Materials with Full-color Tunability from Pure Organic Amorphous Polymers. Sci. Adv. 2022, 8, eabl8392. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ma, H.; Wang, X.; Wang, H.; Wang, Q.; Zou, X.; Song, Z.; Jia, W.; Li, Y.; Mao, Y. Ultralong Organic Phosphorescence from Isolated Molecules with Repulsive Interactions for Multifunctional Applications. Nat. Commun. 2022, 13, 4890. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Chen, B.; He, X.; Li, X.; Wei, P.; Gao, P.F.; Zhang, G.; Lam, J.W.; Tang, B.Z. Highly Efficient and Persistent Room Temperature Phosphorescence from Cluster Exciton Enables Ultrasensitive off-on VOC Sensing. Matter 2022, 5, 3499–3512. [Google Scholar] [CrossRef]
- Tang, L.; Zan, J.; Peng, H.; Yan, X.; Tao, Y.; Tian, D.; Yang, Q.; Li, H.; Chen, Q.; Huang, W. X-ray Excited Ultralong Room-Temperature Phosphorescence for Organic Afterglow Scintillators. Chem. Commun. 2020, 56, 13559–13562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, H.; Yang, J.; Fang, M.; Ding, D.; Tang, B.Z.; Li, Z. High Performance of Simple Organic Phosphorescence Host-guest Materials and Their Application in Time-resolved Bioimaging. Adv. Mater. 2021, 33, 2007811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, Z.; Lam, J.W.; Peng, Q.; Ma, H.; Shuai, Z.; Bai, G.; Hao, J.; Tang, B.Z. Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-temperature Phosphorescence. Chem 2016, 1, 592–602. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, X.; Xie, Y.; Liu, S.; Dong, M.; Zhao, J.; Liang, F.; An, Z.; Huang, W. Recent Advances in Organic Room-Temperature Phosphorescence of Heteroatom (B/S/P)-containing Chromophores. CCS Chem. 2023, 5, 292–309. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Ji, Y.; Chen, R.; Zhu, Q.; Wang, Y.; Zheng, C.; Tao, Y.; Fan, Q.; Huang, W. Invoking Ultralong Room Temperature Phosphorescence of Purely Organic Compounds through H-aggregation Engineering. Mater. Horiz. 2019, 6, 1259–1264. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, L.; Peng, Q.; Fan, D.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Crystallization-induced Dual Emission from Metal-and Heavy Atom-free Aromatic Acids and Esters. Chem. Sci. 2015, 6, 4438–4444. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ge, Y.; Peng, Q.; Li, C.; Li, Q.; Li, Z. How the Molecular Packing Affects the Room Temperature Phosphorescence in Pure Organic Compounds: Ingenious Molecular Design, Detailed Crystal Analysis, and Rational Theoretical Calculations. Adv. Mater. 2017, 29, 1606829. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Xu, T.; Li, Y.; Zhang, C.; Wang, B.; Zhang, X.; Zhang, H.; Qiu, Y.; Yang, J.; Wang, D. H-bonding Room Temperature Phosphorescence Materials via Facile Preparation for Water-stimulated Photoluminescent Ink. Molecules 2022, 27, 6482. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, H.; Lei, Y.; Dai, W.; Liu, M.; Zheng, X.; Cai, Z.; Huang, X.; Wu, H.; Ding, D. Guest-host Doped Strategy for Constructing Ultralong-lifetime Near-infrared Organic Phosphorescence Materials for Bioimaging. Nat. Commun. 2022, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Yang, Z.; Xu, C.; Xie, Z.; Jiang, L.; Gu, F.L.; Zhao, J.; Zhang, Y.; Aldred, M.P.; Chi, Z. Two-photon-excited Ultralong Organic Room Temperature Phosphorescence by Dual-Channel Triplet Harvesting. Chem. Sci. 2019, 10, 7352–7357. [Google Scholar] [CrossRef]
- Amemori, S.; Sasaki, Y.; Yanai, N.; Kimizuka, N. Near-infrared-to-visible Photon Upconversion Sensitized by A Metal Complex with Spin-forbidden Yet Strong S0-T1 Absorption. J. Am. Chem. Soc. 2016, 138, 8702–8705. [Google Scholar] [CrossRef]
- McClure, D.S.; Blake, N.W.; Hanst, P.L. Singlet-triplet Absorption Bands in Some Halogen Substituted Aromatic Compounds. J. Chem. Phys. 1954, 22, 255–258. [Google Scholar] [CrossRef]
- Meyer, D.L.; Lombeck, F.; Huettner, S.; Sommer, M.; Biskup, T. Direct S0→T Excitation of a Conjugated Polymer Repeat Unit: Unusual Spin-forbidden Transitions Probed by Time-resolved Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. Lett. 2017, 8, 1677–1682. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, R.; Tang, X.; Tao, Y.; Xu, S.; Jin, L.; Chen, C.; Zhou, X.; Zheng, C.; Huang, W. Direct Population of Triplet Excited States through Singlet–triplet Transition for Visible-light Excitable Organic Afterglow. Chem. Sci. 2019, 10, 5031–5038. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Z.; Chong, K.C.; Batsanov, A.S.; Yang, Z.; Mao, Z.; Yang, Z.; Liu, B. Carbazole Isomers Induce Ultralong Organic Phosphorescence. Nature Mater. 2021, 20, 175–180. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Zhang, X.; Ou, D.; Mu, Y.; Zhang, Y.; Zhao, C.; Liu, S.; Chi, Z.; Xu, J. Intermolecular Electronic Coupling of Organic Units for Efficient Persistent Room-temperature Phosphorescence. Angew. Chem. Int. Ed. 2016, 55, 2181. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Y.; Wu, X.; Lei, Y.; Zhou, Y.; Cai, Z.; Liu, M.; Wu, H.; Huang, X.; Dong, Y. Excitation-dependent Triplet–singlet Intensity from Organic Host-guest Materials: Tunable Color, White-light Emission, and Room-temperature Phosphorescence. J. Phys. Chem. Lett. 2021, 12, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, S.; Sun, L.; Wu, S.; Hu, G.; Pang, X.; Smith, A.T.; Hu, C.; Zeng, S.; Wang, W. Ultralong Lifetime and Efficient Room Temperature Phosphorescent Carbon Dots through Multi-confinement Structure Design. Nat. Commun. 2020, 11, 5591. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, Y.; Tang, D.; Xiao, H.; Shang, K.; Zhou, X.; Tan, G. Near-Infrared-II Nanoparticles for Vascular Normalization Combined with Immune Checkpoint Blockade via Photodynamic Immunotherapy Inhibit Uveal Melanoma Growth and Metastasis. Adv. Sci. 2023, 10, 2206932. [Google Scholar] [CrossRef]
- Kwon, M.S.; Yu, Y.; Coburn, C.; Phillips, A.W.; Chung, K.; Shanker, A.; Jung, J.; Kim, G.; Pipe, K.; Forrest, S.R. Suppressing Molecular Motions for Enhanced Room-temperature Phosphorescence of Metal-free Organic Materials. Nat. Commun. 2015, 6, 8947. [Google Scholar] [CrossRef]
- Peng, Q.; Niu, Y.; Shi, Q.; Gao, X.; Shuai, Z. Correlation Function Formalism for Triplet Excited State Decay: Combined Spin–orbit and Nonadiabatic Couplings. J. Chem. Theory Comput. 2013, 9, 1132–1143. [Google Scholar] [CrossRef]
- Yang, J.; Zhen, X.; Wang, B.; Gao, X.; Ren, Z.; Wang, J.; Xie, Y.; Li, J.; Peng, Q.; Pu, K. The Influence of the Molecular Packing on the Room Temperature Phosphorescence of Purely Organic Luminogens. Nat. Commun. 2018, 9, 840. [Google Scholar] [CrossRef]
- Jackson, N.E.; Savoie, B.M.; Kohlstedt, K.L.; Olvera De La Cruz, M.; Schatz, G.C.; Chen, L.X.; Ratner, M.A. Controlling Conformations of Conjugated Polymers and Small Molecules: The Role of Nonbonding Interactions. J. Am. Chem. Soc. 2013, 135, 10475–10483. [Google Scholar] [CrossRef]
- Zhou, J.; Stojanović, L.; Berezin, A.A.; Battisti, T.; Gill, A.; Kariuki, B.M.; Bonifazi, D.; Crespo-Otero, R.; Wasielewski, M.R.; Wu, Y.-L. Organic Room-temperature Phosphorescence from Halogen-bonded Organic Frameworks: Hidden Electronic Effects in Rigidified Chromophores. Chem. Sci. 2021, 12, 767–773. [Google Scholar] [CrossRef]
- Su, Y.; Phua, S.Z.F.; Li, Y.; Zhou, X.; Jana, D.; Liu, G.; Lim, W.Q.; Ong, W.K.; Yang, C.; Zhao, Y. Ultralong Room Temperature Phosphorescence from Amorphous Organic Materials toward Confidential Information Encryption and Decryption. Sci. Adv. 2018, 4, eaas9732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% Internal Quantum Efficiency in Undoped Electroluminescent Devices Employing Pure Organic Emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef] [PubMed]





| Comp. | Fluorescence | Organic Afterglow 1 | Organic Afterglow 2 | |||||
|---|---|---|---|---|---|---|---|---|
| λ (nm) | τ (ns) | λ (nm) | τ (ms) | φ (%) | λ (nm) | τ (ms) | φ (%) | |
| PhCz | 380 | 11.2 | 548 | 240 | 1.50 | 548 | 260 | 1.80 |
| PC2Br | 380 | 4.3 | 526 | 63 | 0.37 | 526 | 157 | 5.81 |
| PC3Br | 390 | 2.1 | 536 | 60 | 0.93 | 536 | 70 | 4.38 |
| PC27DBr | 397 | 2.4 | 572 | 50 | 0.56 | 572 | 88 | 3.36 |
| PC36DBr | 404 | 3.3 | 571 | 48 | 0.71 | 571 | 66 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wang, Y.; Zhou, B.; Xie, W.; Zheng, B.; Zhang, J.; Li, P.; Yu, T.; Qi, Y.; Tao, Y.; et al. Direct Population of Triplet States for Efficient Organic Afterglow through the Intra/Intermolecular Heavy-Atom Effect. Molecules 2024, 29, 1014. https://doi.org/10.3390/molecules29051014
Yuan J, Wang Y, Zhou B, Xie W, Zheng B, Zhang J, Li P, Yu T, Qi Y, Tao Y, et al. Direct Population of Triplet States for Efficient Organic Afterglow through the Intra/Intermolecular Heavy-Atom Effect. Molecules. 2024; 29(5):1014. https://doi.org/10.3390/molecules29051014
Chicago/Turabian StyleYuan, Jie, Yongrong Wang, Binbin Zhou, Wenjing Xie, Botao Zheng, Jingyu Zhang, Ping Li, Tian Yu, Yuanyuan Qi, Ye Tao, and et al. 2024. "Direct Population of Triplet States for Efficient Organic Afterglow through the Intra/Intermolecular Heavy-Atom Effect" Molecules 29, no. 5: 1014. https://doi.org/10.3390/molecules29051014
APA StyleYuan, J., Wang, Y., Zhou, B., Xie, W., Zheng, B., Zhang, J., Li, P., Yu, T., Qi, Y., Tao, Y., & Chen, R. (2024). Direct Population of Triplet States for Efficient Organic Afterglow through the Intra/Intermolecular Heavy-Atom Effect. Molecules, 29(5), 1014. https://doi.org/10.3390/molecules29051014